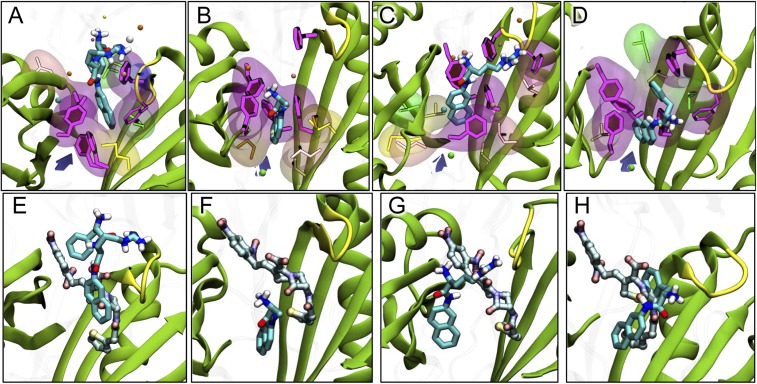
Fig. 5.
Binding of aminoacyl-naphthylamides after 320 ns of MD simulation. (A–D) Binding of PAβN (27) and Ala-, Arg-, and Phe-Naph. The ligand is shown with thick sticks colored by atom type. Side-chains of residues F136, F178, F610, F615, and F628 lining the hydrophobic trap are shown with magenta sticks and with filled rings. Side-chains of hydrophobic residues within 3.5 Å of the ligand are shown both with thinner sticks and with transparent surface. Nonpolar residues are shown with glossy beads. Subdomains PC1/PC2 and the G-loop are shown as cartoon, colored green and yellow, respectively. The entrance from the PC1/PC2 cleft is roughly indicated by a violet arrow. (E–H) Comparison of the most stable poses of PAβN and Ala-, Arg-, and Phe-Naph, with that of nitrocefin, taken from ref. 8. The modulators are shown with sticks colored as in A–D, whereas nitrocefin is shown with carbons in lighter blue.