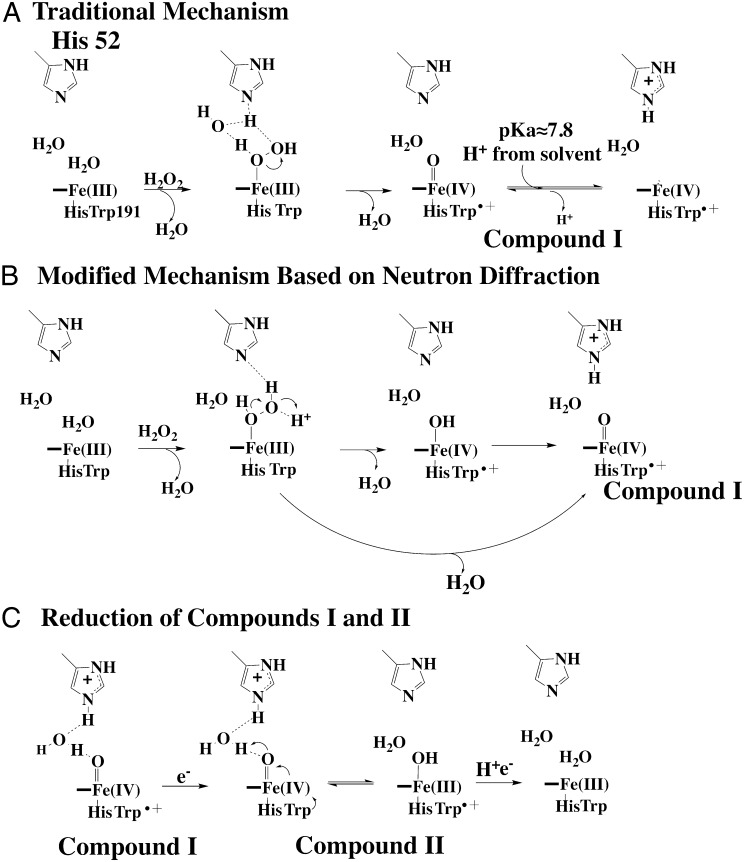
Fig. 1.
Peroxidase mechanism. (A) Traditional “water-modified” mechanism of CmpI formation. In this mechanism, peroxide first coordinates with the heme iron, followed by proton transfer to the distal peroxide O atom via an ordered water molecule and the distal His. The protonation state of the distal His depends on the His pKa. Our computational experiments indicate that the pKa substantially increases in CmpI. (B) Modified mechanism based on the observation that His52 in CCP CmpI is protonated in the neutron diffraction structure (8). Going from the initial peroxide complex to CmpI proceeds via two possible routes, one of which involves the Fe(IV)-OH intermediate. (C) Mechanism of CmpII reduction, which includes a proton-coupled electron transfer event resulting in a net transfer of a proton from the distal His to the ferryl O atom.