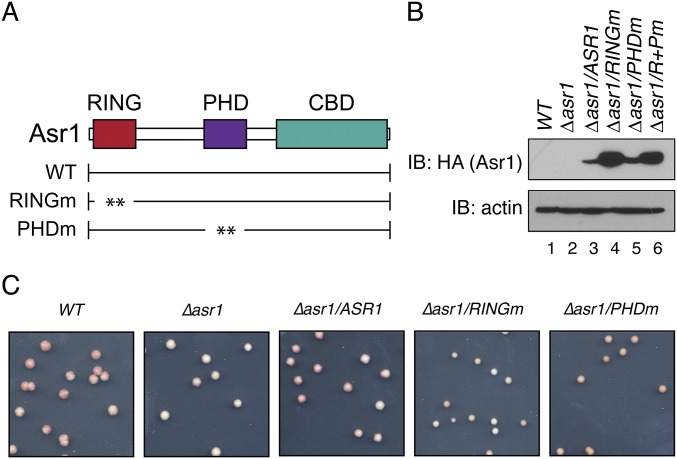
Fig. S1.
The Ub-ligase activity of Asr1 is required for silencing of a telomere-proximal ADE2 reporter. (A) Schematic of Asr1, showing the location of the RING and PHD fingers, and the CTD-binding domain (CBD). The RINGm mutation is a simultaneous substitution of cysteine residues 26, 29, 66, and 69 to alanine. The PHDm mutation is a simultaneous substitution of cysteine residues 143, 146, 186, and 189 to alanine. (B) Steady-state levels of HA-tagged Asr1 proteins assayed in Fig. 1B. Immunoblot (IB) was performed using an anti-HA antibody. An antibody against actin was used as a loading control. Note that the double RING/PHD mutant of Asr1 (R+Pm) was not included in functional assays. (C) Photograph of representative colonies used to calculate colony color ratios presented in Fig. 1B.