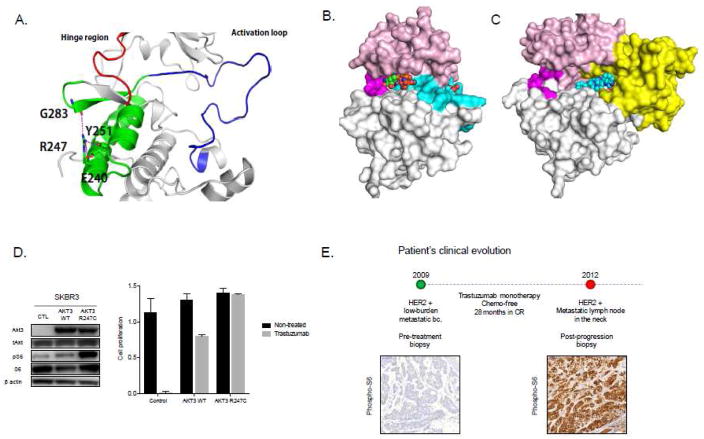
Figure 3.
A novel AKT3 bona fide gain-of-function mutation involved in resistance to trastuzumab monotherapy. A. Cartoon representation of the structure of AKT3 with regions involved in modulating functional conformational changes; residues R247 and those involved in establishing hydrogen bonds (dashed lines) are highlighted in stick representation. B. Structural model of AKT3 built based on homology with AKT1 (88% sequence similarity with the kinase domain and 85% sequence similarity with the PH domain) using the crystal structures of the active state (PDB code 3CQU resolved at 2.2A; the active state does not contain the PH domain) and the inactive state (PDB code 3O96 resolved at 2.7A). AKT3 in its active state with the ATP shown on the left and the phosphorylated Tyr shown on the right. C. AKT3 in its inactive state with the allosteric inhibitor ICQ shown in spheres bound between the kinase and the PH domains. The domains are colored as: pink for the N-terminal lobe, grey for the C-terminal lobe, magenta for the hinge region, cyan for the activation loop and yellow for the PH domain. The activation loop is not highlighted in the inactive state for clarity. D. Transduction of AKT3R247C and AKT3WT coding vectors in ERBB2-amplified SkBr-3 cells. AKT3R247C enhances mTORC1 signaling (left panel) and increases resistance to trastuzumab as seen in colony-formation assays comparing cell proliferation of control, AKT3WT and AKT3R247C transfected cells in the presence of the drug over 7 days. E. The ERBB2-amplified breast cancer patient experienced tumor progression after 28 months in complete response and showed re-activation of mTORC1 activity concurrent with the acquisition of the AKT3R247C mutation.