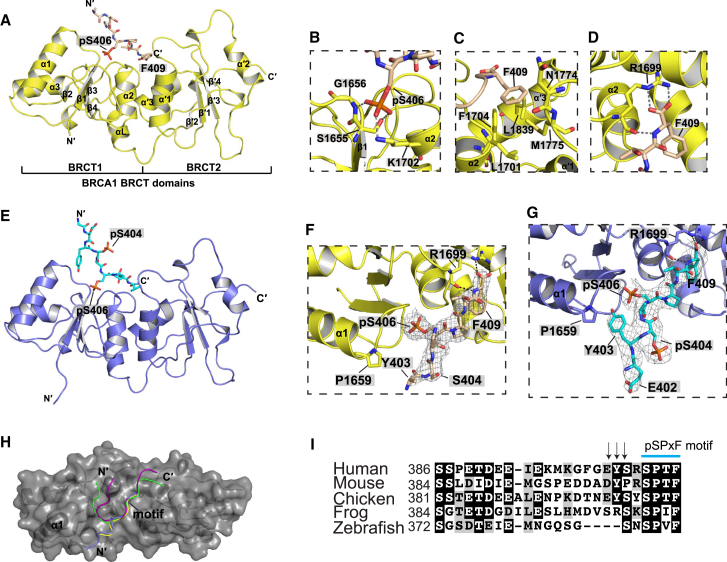
Figure 2.
Crystal Structures of BRCT in Complex with Single and Double Phosphorylated Abraxas Peptide
(A) Crystal structure of BRCT-Ab1p_short. The BRCT domains are in yellow, and the Ab1p_short peptide is in wheat color.
(B–D) Show the detailed interactions between phosphopeptide and BRCT domains. The polar interaction is indicated in dashed lines.
(E) Crystal structure of BRCT-Ab2p. The BRCT is in blue, and the Ab2p peptide is in cyan.
(F and G) Show the interface between BRCT and Ab in both BRCT-Ab1p_short and BRCT-Ab2p structures. The 2Fo-Fc electron density (σ = 1.0) is shown for Abs.
(H) Superimposition of BRCT-Ab2p, BRCT-Ab1p_short, BRCT-Bach1 (PDB code: 1T29), and BRCT-CtIP (PDB code: 1Y98). The BRCT domains are shown in a gray surface representation. Ab2p, Ab1p_short, Bach1, and CtIP are in blue, yellow, green, and purple, respectively. The pSPxF motif is indicated in the image.
(I) Sequence alignment of Abraxas C terminus. The BRCT-binding motif is indicated by a blue line, and the black arrows indicate the half conserved residues (see also Figures S2 and S3).