Fig. 1.
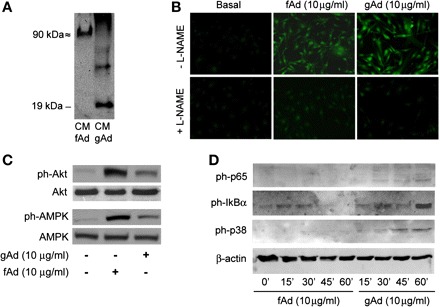
Overlapping and distinct effects of globular (gAd) and full-length adiponectin (fAd) in human aortic endothelial cells (HAEC). A: electrophoresis mobility and oligomerization status of gAd and fAd. Fifty microliters of conditioned media (CM) collected from HAEC treated with fAd or gAd (10 μg/ml, 1 h) was loaded on a 12% nonreducing SDS-PAGE, and membranes were probed with anti-adiponectin antibody. A 90-kDa molecular mass band was found in the CM of fAd-treated HAEC cells. Conversely, a 19-kDa molecular mass band was detected in HAEC treated with gAd. B: HAEC were serum starved (3 h) and loaded with the nitric oxide (NO)-specific fluorescent dye 4,5-diaminofluorescein diacetate (3 μM; Cayman Chemical, Ann Arbor, MI) in the absence or presence of NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 100 μM). HAEC were then stimulated with fAd or gAd (10 mg/ml, 10 min), fixed in 2% paraformaldehyde (PFA) for 5 min at 4°C, and then viewed using an epifluorescent microscope. Emission of green light (510 nm) from cells excited at 480 nm is indicative of NO production. Representative images are shown for experiments that were repeated independently 3 times. C: lysates from HAEC stimulated with fAd or gAd (10 mg/ml, 10 min) were subjected to immunoblotting for phosphorylated (ph) and total forms of Akt and AMP-activated protein kinase (AMPK) proteins. Both fAd and gAd increased phosphorylation levels of Akt and AMPK. D: time course experiments (0, 15, 30, 45, and 60 min) indicate that increased phosphorylation of p65, IκBα, and p38 MAPK in response to treatment with gAd peaks after 1-h stimulation. Treatment with fAd did not significantly enhance phosphorylation levels for NF-κB or p38 MAPK at any of the time points considered.
