Fig. 2.
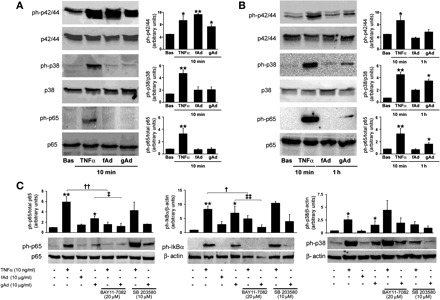
Differential activation of proinflammatory signaling by gAd and fAd in HAEC. HAEC cultured as described in materials and metthods were treated with TNFα (10 ng/ml), fAd (10 μg/ml), or gAd (10 μg/ml) for the indicated time points. HAEC lysates were subjected to immunoblotting with antibodies for total and phosphorylated forms of p42/44 MAPK, p38 MAPK, p65, and IκBα. A: representative immunoblots for results obtained in HAEC stimulated with TNFα (10 ng/ml), fAd (10 μg/ml), or gAd (10 μg/ml) for 10 min. Each bar represents the mean ± SE of densitometric analysis for phosphorylated proteins normalized to their respective total forms. B: representative immunoblots for results obtained in HAEC stimulated with TNFα (10 ng/ml) for 10 min and fAd (10 μg/ml) or gAd (10 μg/ml) for 1 h. Each bar represents the mean ± SE of densitometric analysis for phosphorylated proteins normalized to their respective total forms. C: experiments described in B were repeated in the absence or presence of NF-κB inhibitor BAY 11-7082 (20 μM, 1-h preincubation) or p38 MAPK inhibitor SB-203580 (10 μM, 1-h preincubation). Representative immunoblots from at least 3 independent experiments are shown for each condition. Each bar represents the mean ± SE of densitometric analysis for phosphorylated proteins normalized to their respective total forms. *P < 0.05, **P < 0.01 vs. basal (Bas) conditions; †P < 0.05, ††P < 0.01 vs. TNFα; ‡P < 0.05, ‡‡P < 0.01 vs. gAd.
