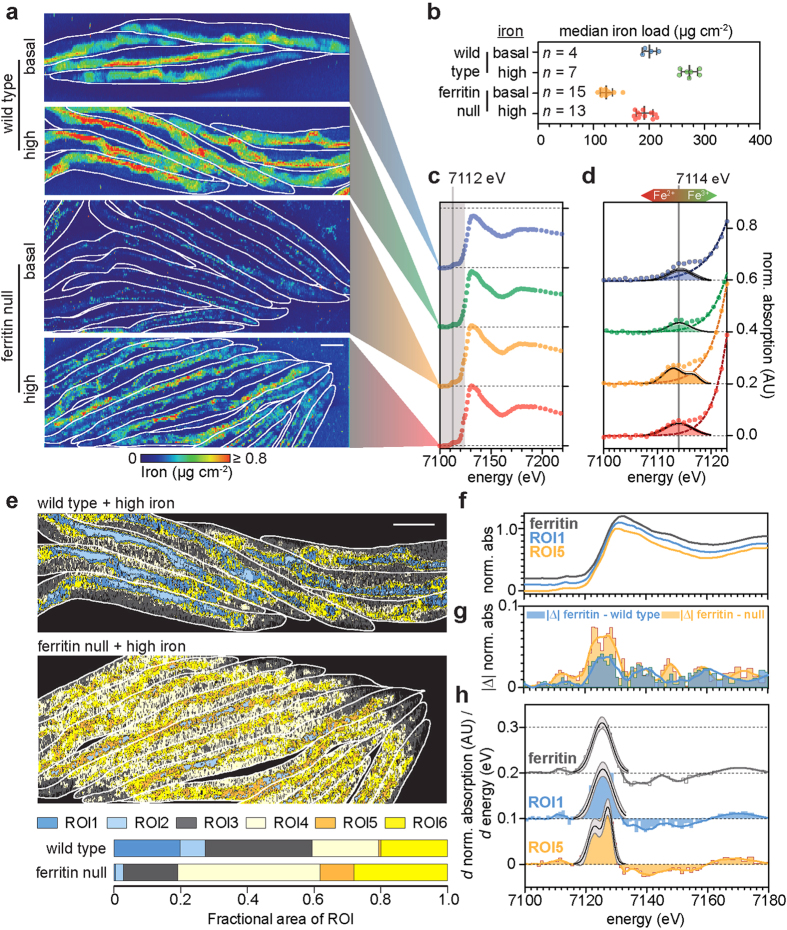
Figure 2. Loss of ferritin skews iron load and Fe(III):total iron ratio.
(a) XFM of wild type and ferritin nulls ± high iron. Ferritin nulls exhibited reduced total iron, but, as expected, retained capacity to uptake iron via a mechanism independent of ferritin. White outline demarcates the boundary of each animal, color table defines iron areal density (μg cm−2) and the scale bar = 100 μm. (b) Median iron areal density for each specimen, showing elevated iron load following exposure to high iron (n = number of specimens per group; data presented as the mean of the medians ± 1 SD). (c) Iron XANES spectra (across all pixels) extracted from low dose φXANES for the groups shown in (a). The starting position of iron K-edge (7112 eV) is marked with a vertical line and for clarity the integrated XANES spectra from each group has been offset vertically. (d) Expanding the pre-edge region (grey box in (c)), following subtraction of the rising edge (dashed line), highlights changes in both the energy and intensity of the 1s → 3d pre-edge feature between groups. The extracted data (colored circles) and fitted Gaussian (solid black lines; 95% confidence interval in grey) are superimposed to determine the centroid values (~7114 eV for wild type; marked for reference). Loss of ferritin changed the pre-edge feature to exhibit two centroid energies (7113 eV and 7117 eV), whereas high iron exposure retained a single centroid energy of 7114 eV. (e) Areas of similar iron XANES spectra identified via principal component analysis and k-means clustering marked as distinct regions of interest (ROIs, six per specimen). The XANES spectra for each cluster were highly structured and allowed the Fe(III):total iron ratio to be calculated for each ROI. The spatial extent of each region as a proportion of the area scanned is shown and highlights that, with the exception of portions of the intestine, the majority of wild type tissues possess relatively low Fe(II) levels despite a higher iron load. In particular, two regions differed significantly in Fe(III):total iron ratio (ROIs 1 and 5; both localized along the intestinal tract) between wild type and ferritin nulls. Scale bar = 100 μm. (f) XANES from a purified horse spleen ferritin standard was compared to the cumulative XANES spectra from ROIs 1 and 5. (g) The difference (ΔXANES) between these two ROIs and the ferritin standard spectra showed that ROI1 had stark similarities with the ferritin profile, whilst ROI5, which was practically absent in wild types demonstrated significant variation from the ferritin XANES spectra, further supporting complete ablation of ferritin from these animals and an altered coordination environment. (h) Features characteristic of electronic transitions used to differentiate between iron oxidation states also revealed that ROI5 had a greater level of abundant Fe(II) compared to ROI1 and the ferritin standard, where the majority of iron is stabilized in a mineralized Fe(III) form.