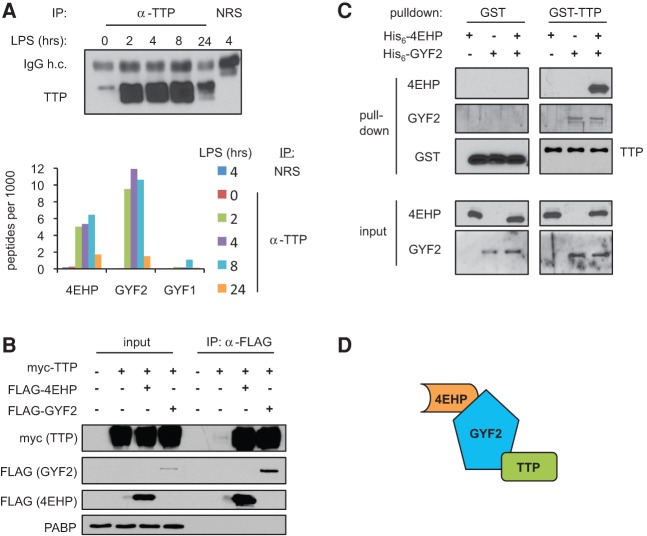
FIGURE 1.
TTP interacts with the 4EHP-GYF2 complex. (A, top) Western blot for TTP in samples immunoprecipitated with anti-TTP or normal rabbit serum (NRS) from mouse macrophage RAW264.7 cells treated with lipopolysaccharide (LPS) for various lengths of time as indicated. (IgG h.c.) IgG heavy chain. (Bottom) Graph showing the number of peptides for 4EHP, GYF2, and GYF1 detected per 1000 of total detected peptides in LC-MS/MS analyses of the IPs. (B) Western blots of input and anti-Flag IPs from HEK293T cells cotransfected with myc-TTP and Flag-4EHP or Flag-GYF2. Lysates were treated with RNase A prior to IP. (C) Western blots monitoring His6-4EHP and His6-GYF2 interaction with GST or GST-TTP in in vitro pull-down assays using glutathione Sepharose beads. GST and GST-TTP were detected using anti-GST; 4EHP and GYF2 were detected using anti-4EHP and anti-GYF2, respectively. (D) Proposed model of interaction between TTP and the 4EHP-GYF2 complex. TTP directly binds GYF2, which is known to interact with 4EHP.