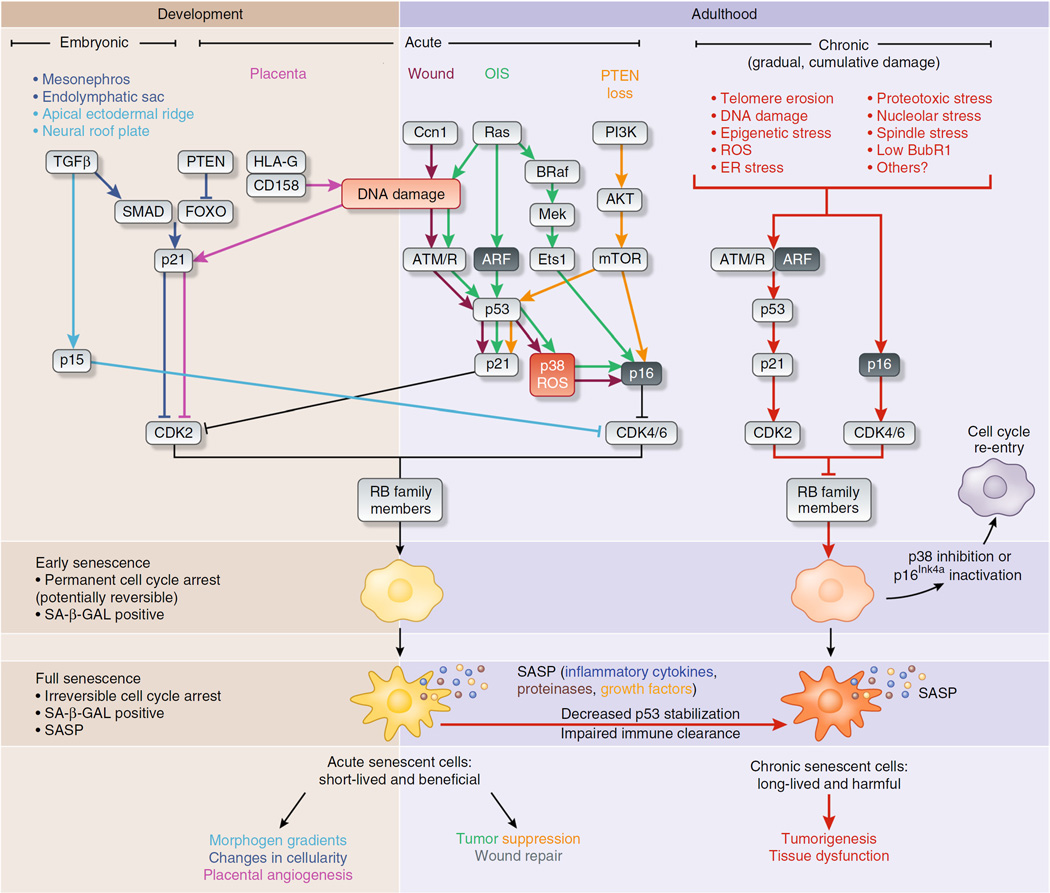
Figure 1.
Effector pathways of three senescent cell types. Stresses inducing senescence vary depending on the in vivo context, although there is substantial overlap in processing of the stress-response signal and activating effectors of senescence. For example, in all reported cases, rising levels of cyclin-dependent kinase inhibitors drive entry into senescence by activating RB to block cell cycle progression. In embryonic senescence, elevated TGFβ and reduced PTEN activity upregulate SMAD-FOXO transcriptional activation of the cyclin-dependent kinase inhibitor p21, as well as activation of p15Ink4b through unclear means. In contrast, acute senescence—in the placenta, after wounding or in response to oncogene activation or loss of the tumor suppressor PTEN—triggers DNA damage or p53 signaling to induce p21 and p16Ink4a. Both embryonic and acute senescence are beneficial, and presumably these cells are cleared rapidly by the immune system as part of their program. These two settings contrast with chronic senescence, which is a response to the slowly accumulating macromolecular damage of age, such as telomere erosion, proteotoxicity, DNA damage and likely many others. Effectors of chronic senescence probably include p21 and p16Ink4a, which are induced in aged tissues. Chronic senescence can also evolve from acute senescence if immune clearance is impaired with age, leading to prolonged arrest and possibly alterations in the SASP. In all senescence cases, cyclin-dependent kinase–mediated licensing of RB activity leads to an early senescent state where the arrest is permanent in vivo, but can be reversed with manipulation of single factors, such as p38 inhibition or inactivation of p16Ink4a. These early senescent cells are SA-β-GAL positive and may not have a SASP. Senescent cells may evolve further into a truly irreversible full senescence with SA-β-GAL positivity and a SASP. The cellular changes driving this phenotypic switch in vivo are unclear but are likely to include robust processes such as heterochomatinization of cell cycle genes and activation of an NF-κB–dependent transcriptional program generating the SASP.