Fig 2.
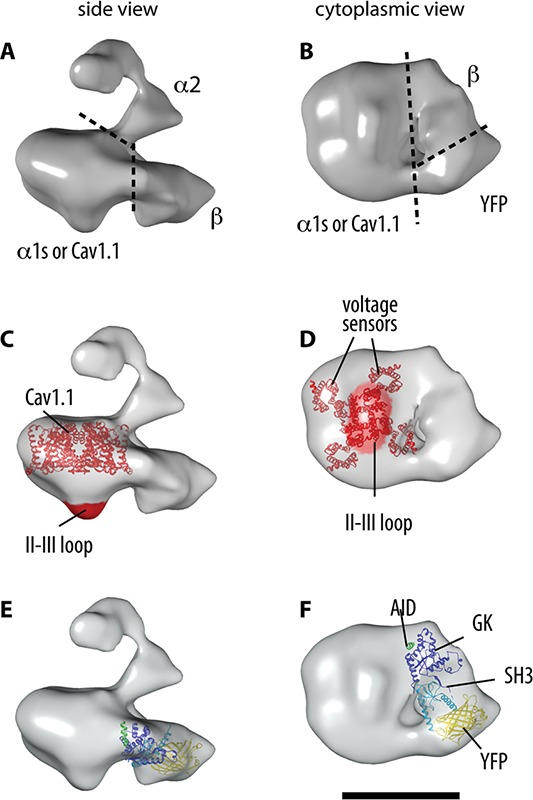
3D reconstruction of the DHPR and docking of the α1s and β subunits. A-B. The boundaries of the oc1s, α2 and β subunits within the 3D reconstruction of the DHPR are indicated in the two orthogonal views. C-D. Molecular boundaries of α1s: docking of the atomic structure of a representative voltage-gated cation channel (NavAb32), and location of the II-III loop. E-F. Docking of the crystal structure of a β subunit35 with the SH3 and GK domains indicated in light and dark blue, respectively. The barrel-shape of YFP (yellow), engineered in the N terminus of β1a, is recognizable in the 3D structure. The AID sequence of α1s’ I-II loop (green) that co-crystallized with the β subunits makes contact with the α1s subunit. According to functional studies, the γ subunit should locate at the interphase between α1s and β. Scale bar, 10 nm.
