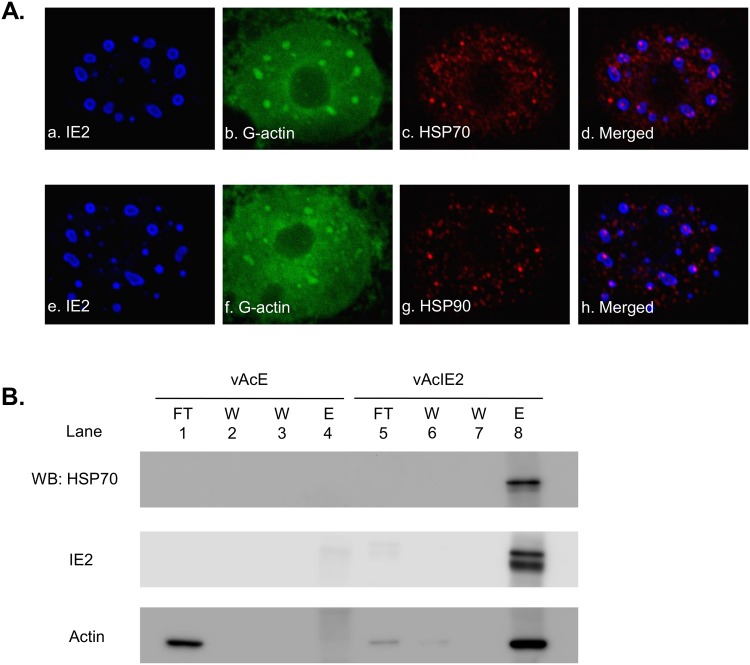
Fig 2. IE2 associates with HSPs.
vAcIE2-transduced Vero E6 cells were fixed and examined by immunofluorescence staining at 48 hpt. Nuclei have been enlarged to more clearly present the interaction between IE2, HSP70 and HSP90. (A) Panels a to d show that HSP70 formed dots and was scattered within the nucleus. Larger foci of HSP70 closely associated with IE2 nuclear bodies. Panels e to h show that HSP90 also formed foci and was closely associated with IE2 nuclear bodies. (B) The IE2 protein complex was immunoprecipitated using an anti-histidine antibody from nuclear extracts of the vAcIE2-transduced Vero E6 cells. Nuclear extracts of the vAcE-transduced Vero E6 cells were used as a control. HSP70, actin and IE2 were detected by Western blotting using the corresponding antibody. FT: flow-through, W: wash, E: eluted fraction.