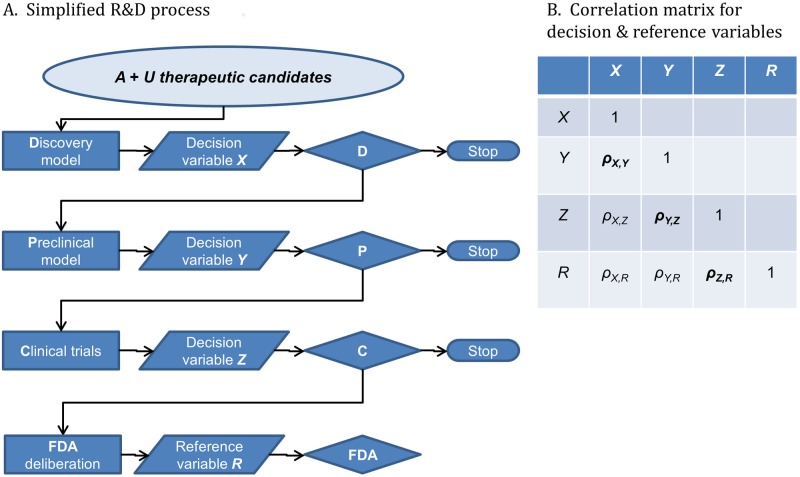
Fig 1. Decision theoretic view of biopharma discovery, research, and development.
(A) The process starts with a large set of therapeutic possibilities (light blue oval). These could be putative disease mechanisms or candidate drug targets, in either an academic or commercial setting. However, we discuss them as if they are molecules in a commercial R&D campaign (e.g., compounds in a screening library and the analogues that could be reasonably synthesized to create leads). There are A candidates that with perfect R&D decision making and an unlimited R&D budget would eventually be approved by the drug regulator for the indication or indications. There are U candidates that would not succeed given similar skill and investment. In general, U >> A. The Discovery (D), Preclinical (P), and Clinical Trial (C) diamonds are “classifiers” (Table 1). Each takes decision variables (X, Y, Z) from predictive models for some or all of the candidates and tests the variables against a decision threshold, yielding yeses which receive further scrutiny or noes which are abandoned. The unit cost per surviving candidate increases through the process [21]. Given serial decisions, only yeses from C face the gold standard reference test; the drug regulator (e.g., the Food and Drug Administration, or FDA). The other decisions face “imperfect” reference tests [33] [34] [27], the next steps in the process, which are mere proxies for the gold standard. The imperfect reference test for yeses from D is provided by P. The imperfect reference test for yeses from P is provided by C. (B) Decision variables X, Y, and Z, will correlate to a greater or lesser extent with each other and with the gold standard reference variable R. The correlation coefficient between X and Y is ρX,Y, the correlation coefficient between Y and Z is ρY,Z, etc. Most of these correlations will never be measured directly during the R&D process. If ρX,R is very low, the Discovery stage will not enrich the Preclinical stage for approvable candidates, even if ρX,Y is high and decisions from D initially appear to have been successful.