Abstract
Purpose:The majority deaths of cancer patients are related to metastasis, thus genes associated with cell motility interest us. SPOCK1 was elected by data mining and serial evaluation. In addition, SPOCK1 has been reported to be highly expressed in different human cancers and been related to adverse outcomes. Therefore, we validate its prognostic significance in urothelial carcinoma (UC).
Materials and Methods:Real-time RT-PCR assay was used to detect SPOCK1 transcript level in 27 urinary tract urothelial carcinoma (UTUC) and 27 urinary bladder urothelial carcinoma (UBUC) samples. Immunohistochemistry evaluated by H-score determined SPOCK1 expressions in 340 UTUCs and 295 UBUCs. The transcript and protein expression were correlated with clinicopathological features. Further evaluations of the prognostic significance of SPOCK1 for disease-specific survival (DSS) and metastasis-free survival (MeFS) were analyzed.
Results:The expressions of SPOCK1 in UC were higher than those in normal urothelium by immunohistochemistry. The statistical analysis of clinicopathologic characteristics and immunohistochemistry showed that the higher expression of SPOCK1 was correlated to pT status (P<0.001), lymph node metastasis (UTUC, P=0.006; UBUC, P=0.033), higher histological grade (UTUC, P<0.001; UBUC, P<0.001), vascular invasion (UTUC, P<0.001; UBUC, P<0.001), perineurial invasion (UTUC, P<0.001; UBUC, P=0.001) and frequent mitosis (UTUC, P<0.001; UBUC, P=0.001). The prognosis of SPOCK1 of UC showed high SPOCK1 expression had significantly worse DSS and MeFS.
Conclusions:The investigation demonstrated that the higher expression of SPOCK1 correlates with a poor prognosis in UC.
Keywords: transcriptome, SPOCK1, urothelial carcinoma, prognosis.
Introduction
Urothelial carcinoma (UC), the predominant type among the varied histologic malignancies arising from the urinary tract, derives from the urothelial lining either in the lower tract (urinary bladder) or the upper tract (renal pelvis, ureter) 1, 2. About 80% of patients with urinary bladder urothelial carcinoma face recurrence within one to two years of initial treatment 3, 4. A significant proportion of these patients die because of recurrence even after receiving standard treatment 5. In addition, around 25% of patients with UC have muscle-invasive disease, and either present with or later develop metastasis 6. Thus effort is needed to explore the development and progression of UC in order to identify a better prognosticator and to enable better oncology-targeted therapies.
The majority deaths among cancer patients result from metastasis rather than the primary disease. Metastasis is the distal settlement of tumor cells and stats escaping from the primary location. Therefore we investigated genes related to cell motility in UC, which have not been systemically evaluated before. From data mining and comparison with other related genes, SPOCK1 demonstrated exciting associations with invasiveness and metastasis in the transcriptome GSE31684.
SPOCK1 encodes a calcium-binding matricellular glycoprotein belonging to the SPARC family 7. The importance of SPARC in regulating proliferation, cell-cycle progression, apoptosis, adhesion, and cell-matrix interaction has been well documented 8. Recent evidence suggests that SPOCK1, which is structurally similar, may play a crucial role in invasion of pilocytic astrocytoma 9 and in progression of hepatocellular carcinoma (HCC) 10. Our study has found that SPOCK1 expression is correlated with adverse clinicopathological factors and survival in a well-characterized cohort of UC.
Materials and Methods
Data mining of the GEO to identify altered transcripts in UC
One dataset, GSE31684 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE31684), which profiled radical cystectomy specimens from 93 urinary bladder urothelial carcinoma (UBUC) cases, was identified by data mining of the GEO (National Center for Biotechnology information, Bethesda, MD, USA) and by Affymetrix U133 Plus 2.0 Array. To evaluate the gene expression level, we imported the raw CEL files into the Nexus Expression 3 statistical software (BioDiscovery, EI Segundo, CA, USA). All probe sets were used in the evaluation without preselection or filtering. We performed supervised comparative analysis to examine the statistical significance of differentially expressed genes on the basis of progression in primary tumor status (pT) and the development of metastatic events. With this intention, we compared the differential expression in low-stage (pTa-pT1) UCs and muscle-invasive, high-stage (pT2-pT4) UCs, and compared non-metastatic lesions to those that developed distal metastasis, respectively, in order to perform functional profiles focusing on the genes related to cell motility (GO:0048870). Only genes displaying significantly differential expression in both stage (log2 ratio > 1.0) and metastasis (log2 ratio > 0.5) were enrolled for initial validation.
Patients and tumor specimens
This study was approved by the Institutional Review Board (IRB) of Chi Mei Medical Center, approval number IRB10302015. All specimens were obtained from the BioBank of Chi Mei Medical Center and had been collected previously following official ethical guidelines. We retrieved urothelial carcinoma cases for immunohistochemical study and survival analysis between 1996 and 2004 from the Chi Mei Medical Center archives. For the initial validation, which focused on identifying the most significant among the candidate genes, we randomly selected 50 UBUCs and 50 urinary tract urothelial carcinomas (UTUCs) as the pilot batch of cases. We further evaluated the gene demonstrating greatest clinical significance in an independent cohort, as previously described 11. The independent cohort included a total of 635 consecutively treated, well-characterized cases: 340 tumors originating from the UT and 295 arising from the UB. All patients received nephroureterectomy and excision of the bladder with regional lymph node dissection. Of these cases, UBUC patients with pT3 or pT4 stage tumors or with nodal involvement received cisplatin-based post-operative adjuvant chemotherapy, but patients with renal insufficiency obtained carboplatin. However, only 29 of the 106 UTUC patients with pT3 or pT4 stage tumors or nodal involvement received post-operative adjuvant chemotherapy. In addition, neo-adjuvant chemotherapy was not introduced to the patients with UBUC or UTUC in our cohort. The criteria for clinicopathological evaluation were essentially identical to those in our previous work 11. Two pathologists (P.I.L & C.F.L) re-evaluated hematoxylin-eosin sections of all cases.
Immunohistochemical staining
Following histological review, tissue blocks containing the most invasive area of each case were selected for immunohistochemical study. We cut representative tissue sections of 4-μm thickness onto precoated slides from the paraffin-embedded tissue blocks, succeeded by deparaffinization, rehydration, antigen retrieval, and blockage of endogenous peroxidase. Endogenous peroxidase was buffered with saline for 15 minutes and subsequently incubated with a primary antibody targeting CALD1 (1:100, C-19, Santa Cruz) or SPOCK1 (1:200, 102D1, Santa Cruz) for one hour. We detected primary antibodies using the DAKO ChemMate EnVision Kit (K5001, Carpinteria, CA, USA). The presence of brown chromogen in the cytoplasm of target cells indicated positive immunoreactivity. The quality of immunostaining was ensured by a negative control incubated without the primary antibody.
Interpretation and scoring of immunohistochemistry
Two pathologists (P.I.L & C.F.L) appraised the immunohistochemistry without prior knowledge of clinical and follow-up data. Immunoreactivity was estimated with a combination of the percentage and intensity of positively stained tumor cytoplasm in order to generate the H-score, which was calculated by the following equation: H-score=∑Pi(i +1), where i is the intensity of stained tumor cells (0 to 3+), and Pi is the percentage of stained tumor cells for each intensity varying from 0% to 100%. This formula produces a score range from 100-400, where 100 equals 100% of tumor cells showing negative results and 400 equals 100% of tumor cells strongly stained (3+) 12, 13.
Real-time RT-PCR
Since SPOCK1 was identified as the most significantly altered gene, its transcript level was further determined in a collection of snap-frozen samples containing high percentage (no less than 70%) of tumor elements consisting of 27 UBUCs and 27 UTUCs, respectively. For this goal, total RNAs were extracted, quantified, and submitted for reverse-transcription. Using pre-designed TaqMan assay reagents (Applied Biosystems), we measured mRNA abundance of SPOCK1 (Hs00270274_m1) with the ABI StepOnePlus™ System. The fold expression of SPOCK1 relative to normal urothelium was calculated by comparative Ct method, after normalization to POLR2A (Hs01108291_m1) as the internal control.
Statistical analysis
Statistical analysis was performed using SPSS V.14.0 software (SPSS Inc. Chicago, IL, USA). The median H-score of immunohistochemistry for SPOCK1 was the cutoff to dichotomize the study cohort, separating cases into high expression and low expression groups. We employed the chi-square test to compare SPOCK1 expression status and various clinicopathological parameters. The end points analyzed were disease-specific survival (DSS) and metastasis-free survival (MeFS), calculated from the date of curative surgery to the date an event developed. Patients lost to follow-up were censored on the final follow-up date. We plotted survival curves using the Kaplan-Meier method, and evaluated prognostic differences between groups using the log-rank test. Parameters demonstrating P values less than 0.05 in univariate analysis were subsequently enrolled into multivariate tests using the Cox proportional hazards model. For all analyses, two-sided tests of significance with P<0.05 were considered significant.
Results
CALD1, SPOCK1, SCG2, IGF1 and CEECAM1 were identified as significant differentially upregulated transcripts implicated in cell motility in UC
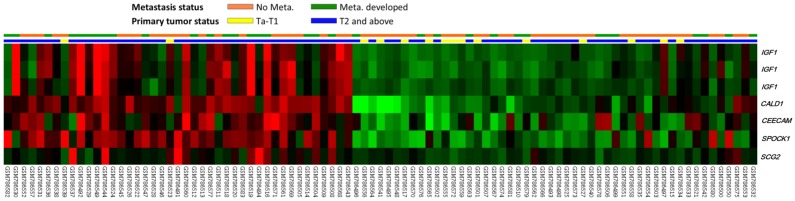
From the transcriptomic profiles of GSE31684, we found 109 probes covering 52 transcripts associated with cell motility (GO:0048870). Of these, only seven probes covering five genes showed significant differential expression associated with both increments of primary tumor stage and the presence of distal metastasis (Fig. 1 and Table 1). Of these, the upregulated CALD1 and SPOCK1 were more significant and met the selection criteria. Of the candidate genes, CALD1 was the most significantly upregulated with increments of primary tumor status, showing a log2 ratio of 1.6569 fold. SPOCK1 upregulation was most significantly associated with the presence of distal metastasis (log2 ratio of 0.843).
Figure 1.
Analysis of gene expression in urothelial carcinomas of the bladders from a published transcriptomic dataset (GSE31684). Clustering analysis of genes focusing on cell motility (GO:0048870) showed upregulation of IGF1, CALD1, CEECAM, SPOCK1 and SCG2 were more significantly associated with both higher primary tumor status (pT) and distal metastasis. Specimens from high pT (blue lines) and low pT (yellow lines) are indicated on top of the heatmap, and upregulation and downregulation of genes are illustrated as a spectrum of brightness of red and green, respectively, with those unaltered coded as black.
Table 1.
Summary of differentially expressed genes associated with Cell Motility (GO: 0048870) and showed positive associations to cancer invasiveness and metastasis in the transcriptome of urothelial carcinoma of urinary bladder (GSE31684).
| Probe | Comparing T2-4 to Ta-T1 | Comparing Meta. to Non-Meta.# | Gene Symbol | Biological Process | Molecular Function | ||
|---|---|---|---|---|---|---|---|
| log ratio | p-value | log ratio | p-value | ||||
| 201617_x_at | 1.6569 | <0.0001 | 0.6623 | 0.0085 | CALD1 | cell motility, muscle contraction | actin binding, calmodulin binding, myosin binding, tropomyosin binding |
| 202363_at | 1.1471 | 0.0003 | 0.843 | 0.0005 | SPOCK1 | cell adhesion, cell motility, cell proliferation, multicellular organismal development, nervous system development | calcium ion binding |
| 204035_at | 0.5489 | 0.0071 | 0.519 | 0.0009 | SCG2 | MAPKKK cascade, angiogenesis, cell motility, endothelial cell migration, eosinophil chemotaxis, induction of positive chemotaxis, inflammatory response, intracellular signaling cascade, negative regulation of apoptosis, negative regulation of endothelial cell proliferation, positive regulation of endothelial cell proliferation, protein secretion | calcium ion binding, chemoattractant activity, cytokine activity |
| 209540_at | 0.7559 | 0.0038 | 0.6879 | 0.0005 | IGF1 | DNA replication, Ras protein signal transduction, cell motility, glycolate metabolic process, muscle development, positive regulation of cell proliferation, regulation of steroid hormone receptor signaling pathway, sensory perception of sound, signal transduction, skeletal development | growth factor activity, hormone activity, insulin-like growth factor receptor binding, prothoracicotrophic hormone activity |
| 209541_at | 0.8353 | 0.0015 | 0.7105 | 0.0005 | IGF1 | DNA replication, Ras protein signal transduction, cell motility, glycolate metabolic process, muscle development, positive regulation of cell proliferation, regulation of steroid hormone receptor signaling pathway, sensory perception of sound, signal transduction, skeletal development | growth factor activity, hormone activity, insulin-like growth factor receptor binding, prothoracicotrophic hormone activity |
| 209542_x_at | 0.3483 | 0.0058 | 0.3631 | 0.0001 | IGF1 | DNA replication, Ras protein signal transduction, cell motility, glycolate metabolic process, muscle development, positive regulation of cell proliferation, regulation of steroid hormone receptor signaling pathway, sensory perception of sound, signal transduction, skeletal development | growth factor activity, hormone activity, insulin-like growth factor receptor binding, prothoracicotrophic hormone activity |
| 224794_s_at | 1.0792 | <0.0001 | 0.4795 | 0.0067 | CEECAM1 | cell adhesion, cell motility, leukocyte adhesion, lipopolysaccharide biosynthetic process | oxidoreductase activity; acting on single donors with incorporation of molecular oxygen; incorporation of two atoms of oxygen, procollagen-lysine 5-dioxygenase activity |
#, Meta., distal metastasis developed during follow-up; Non-Meta.: no metastatic event developed.
SPOCK1 expression was most significantly associated with tumor aggressiveness in genes associated with cell motility
In the pilot batch of cases for initial validation, SPOCK1 but not CALD1 overexpression was significantly associated with both primary tumor status (UTUC, P=0.004; UBUC, P=0.009) and nodal metastasis (UTUC, P=0.037; UBUC, P=0.042) (Table S1). Moreover, SPOCK1 was the most significant candidate that predicted inferior DSS (UTUC, P=0.0002; UBUC, P=0.0017) and worse MeFS (UTUC, P<0.0001; UBUC, P=0.0006) (Table S2).
SPOCK1 mRNA expression is positively associated with higher pT status in both UTUC and UBUC
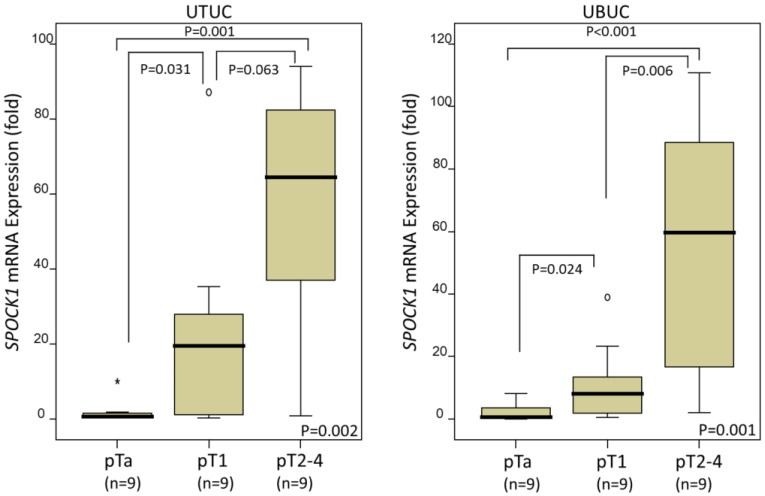
In the 27 UTUCs and 27 UBUCs tested, SPOCK1 mRNA expression was significantly upregulated with higher pT status in both UTUC (P=0.002) and UBUC (P=0.001), suggesting that it plays a role in tumor progression (Fig. 2).
Figure 2.
Quantitative real-time RT-PCR analysis revealed that SPOCK1 mRNA expression was significantly upregulated in urinary tract urothelial carcinomas (UTUCs, left panel) and urinary bladder urothelial carcinomas (UBUCs, right panel) with higher primary tumor status.
Clinicopathological findings for UTUC
The clinicopathological features of the UTUC patients are listed in Table 2. The disease showed no predilection for either sex. The median age at diagnosis was 68 years, ranging from 34 to 87 years. Sixty-two patients (18.2%) suffered from multifocal tumors, and 49 (14.4%) had tumors involving both the renal pelvis and ureter. The majority of cases (n=284, 83.5%) were of high histological grade. Advanced pT stage (pT2-T4) was seen in 159 cases (46.8%). Around one-half of the cases (n=167, 49.1%) showed mitotic activity. Vascular invasion and perineurial invasion were noted in 106 cases (31.2%) and 19 cases (5.9%), respectively. Nodal metastasis was observed in 28 patients (8.2%).
Table 2.
Correlations between SPOCK1 Expression and other important clinicopathological parameters in urothelial carcinomas.
| Parameter | Category | Upper Urinary Tract Urothelial Carcinoma | Urinary Bladder Urothelial Carcinoma | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Case No. | SPOCK1 Expression | P value | Case No. | SPOCK1 Expression | P value | ||||
| Low | High | Low | High | ||||||
| Gender& | Male | 158 | 82 | 76 | 0.514 | 216 | 105 | 111 | 0.489 |
| Female | 182 | 88 | 94 | 79 | 42 | 37 | |||
| Age (years)# | 340 | 65.16±10.91 | 66.00±8.76 | 0.961 | 295 | 65.27±13.02 | 66.81±11.35 | 0.543 | |
| Tumor location& | Renal pelvis | 141 | 66 | 75 | 0.194 | - | - | - | - |
| Ureter | 150 | 83 | 67 | - | - | - | - | ||
| Renal pelvis & ureter | 49 | 21 | 28 | - | - | - | - | ||
| Multifocality& | Single | 278 | 143 | 135 | 0.261 | - | - | - | - |
| Multifocal | 62 | 27 | 35 | - | - | - | - | ||
| Primary tumor (T) & | Ta | 89 | 61 | 28 | <0.001* | 84 | 70 | 14 | <0.001* |
| T1 | 92 | 57 | 35 | 88 | 42 | 46 | |||
| T2-T4 | 159 | 52 | 107 | 123 | 35 | 88 | |||
| Nodal metastasis& | Negative (N0) | 312 | 163 | 149 | 0.006* | 266 | 138 | 128 | 0.033* |
| Positive (N1-N2) | 28 | 7 | 21 | 29 | 9 | 20 | |||
| Histological grade & | Low grade | 56 | 41 | 15 | <0.001* | 56 | 46 | 10 | <0.001* |
| High grade | 284 | 129 | 155 | 239 | 101 | 138 | |||
| Vascular invasion& | Absent | 234 | 148 | 86 | <0.001* | 246 | 140 | 106 | <0.001* |
| Present | 106 | 22 | 84 | 49 | 7 | 42 | |||
| Perineural invasion& | Absent | 321 | 169 | 152 | <0.001* | 275 | 144 | 131 | 0.001* |
| Present | 19 | 1 | 18 | 20 | 3 | 17 | |||
| Mitotic rate (per 10 high power fields)# | 340 | 10.63±12.07 | 13.99±12.30 | <0.001* | 295 | 12.41±13.89 | 16.39±13.95 | 0.001* | |
&, Chi-Square test; #, Mann-Whitney U test; * Statistically significant.
Clinicopathological findings for UBUC
UBUC patients were predominantly male (n=216, 73.2%) and older than 65 years (n=174, 59.0%). As outlined in Table 2, most (n=239, 81%) were of high histological grade, with 123 (41.7%) in advanced stages (pT2-T4) at initial diagnosis. One hundred and fifty-six cases (52.9%) showed high mitotic activity (≥ 10/10HPFs). Lymph node metastasis was detected in 23.6% of patients (n=29). Vascular invasion was evident in 49 cases (16.6%) and perineurial invasion in 20 cases (6.8%).
Correlation of SPOCK1 immunoreactivity with parameters in UTUC and UBUC
SPOCK1 demonstrates variable cytoplasmic expression in carcinoma cells of both the UT and UB, with median H-scores of 260 (range, 110-380) and 285 (range, 115-375) respectively. In contrast, the expression level of SPOCK1 is very low in normal urothelium, with H-scores ranging from 100 to 140. After dichotomizing the tumors into those with low and high SPOCK1 expression, as illustrated in Table 2, we found increased SPOCK1 expression was significantly associated with increments of pT status (P<0.001), lymph node metastasis (UTUC, P=0.006; UBUC, P=0.033), higher histological grade (UTUC, P<0.001; UBUC, P<0.001), vascular invasion (UTUC, P<0.001; UBUC, P<0.001), perineurial invasion (UTUC, P<0.001; UBUC, P=0.001) and frequent mitosis (UTUC, P<0.001; UBUC, P=0.001) in urothelial carcinomas of the two locations. These findings point to a role for SPOCK1 in the progression of UC.
Survival analysis for UTUC
Follow-up information, with duration ranging from 1 to 176 months (median, 38), was available for all patients. Univariate and multivariate analyses of the association between clinical outcomes and various clinicopathological features of UTUC cases are summarized in Table 3. Both univariate and multivariate analysis revealed that poor DSS was significantly associated with multifocality (P=0.0042 and P=0.006, respectively), advanced pT (P<0.0001 and P=0.010, respectively), lymph node metastasis (P<0.0001 and P<0.0001, respectively), high histological grade (P=0.0171 and P=0.044, respectively), perineurial invasion (P<0.0001 and P=0.002, respectively), and high SPOCK1 expression (P<0.0001 and P=0.031, respectively). Similar results were also seen for MeFS, excluding primary tumor and histological grade. However, vascular invasion in UTUC was also independently and significantly associated with worse MeFS (P<0.0001 and P=0.015, respectively).
Table 3.
Univariate log-rank and multivariate analyses for Disease-specific and Metastasis-free Survivals in Upper urinary tract urothelial carcinoma
| Parameter | Category | Case No. | Disease-specific Survival | Metastasis-free Survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| No. of event | P value | R.R. | 95% C.I. | P value | No. of event | P value | R.R. | 95% C.I. | P value | |||
| Gender | Male | 158 | 28 | 0.9301 | - | - | - | 32 | 0.7904 | - | - | - |
| Female | 182 | 33 | - | - | - | 38 | - | - | - | |||
| Age (years) | < 65 | 138 | 26 | 0.8660 | - | - | - | 30 | 0.8470 | - | - | - |
| ≥ 65 | 202 | 35 | - | - | - | 40 | - | - | - | |||
| Tumor side | Right | 177 | 34 | 0.7188 | - | - | - | 38 | 0.3074 | - | - | - |
| Left | 154 | 26 | - | - | - | 32 | - | - | - | |||
| Bilateral | 9 | 1 | - | - | - | 0 | - | - | - | |||
| Tumor location | Renal pelvis | 141 | 24 | 0.0120* | 1 | - | 0.912 | 31 | 0.0659 | - | - | - |
| Ureter | 150 | 22 | 0.794 | 0.421-1.500 | 25 | - | - | - | ||||
| Renal pelvis & ureter | 49 | 15 | 1.641 | 0.452-5.955 | 14 | - | - | |||||
| Multifocality | Single | 273 | 48 | 0.0042* | 1 | - | 0.006* | 52 | 0.0196* | 1 | - | 0.002* |
| Multifocal | 62 | 18 | 2.809 | 1.355-6.162 | 18 | 2.383 | 1.369-4.149 | |||||
| Primary tumor (T) | Ta | 89 | 2 | <0.0001* | 1 | - | 0.010* | 4 | <0.0001* | 1 | - | 0.106 |
| T1 | 92 | 9 | 1.832 | 0.976-4.202 | 15 | 2.641 | 0.844-8.264 | |||||
| T2-T4 | 159 | 50 | 6.173 | 1.359-27.78 | 51 | 2.867 | 0.905-9.082 | |||||
| Nodal metastasis | Negative (N0) | 312 | 42 | <0.0001* | 1 | - | <0.001* | 55 | <0.0001* | 1 | - | 0.001* |
| Positive (N1-N2) | 28 | 19 | 4.971 | 2.657-9.301 | 15 | 2.932 | 1.575-5.457 | |||||
| Histological grade | Low grade | 56 | 4 | 0.0171* | 1 | - | 0.044* | 3 | 0.0019* | 1 | - | 0.058 |
| High grade | 284 | 57 | 3.182 | 1.034-9.788 | 67 | 3.288 | 0.961-11.249 | |||||
| Vascular invasion | Absent | 234 | 24 | <0.0001* | 1 | - | 0.351 | 26 | <0.0001* | 1 | - | 0.015* |
| Present | 106 | 37 | 1.337 | 0.726-2.463 | 44 | 2.184 | 1.162-4.105 | |||||
| Perineural invasion | Absent | 321 | 50 | <0.0001* | 1 | - | 0.002* | 61 | <0.0001* | 1 | - | 0.018* |
| Present | 19 | 11 | 3.335 | 1.556-7.150 | -- | 9 | 2.485 | 1.172-5.210 | ||||
| Mitotic rate (per 10 high power fields) | < 10 | 173 | 27 | 0.1268 | - | - | - | 30 | 0.0581 | - | - | - |
| >= 10 | 167 | 34 | - | - | - | 40 | - | - | - | |||
| SPOCK1 expression | Low | 170 | 10 | <0.0001* | 1 | - | 0.031* | 14 | <0.0001* | 1 | - | 0.039* |
| High | 170 | 50 | 2.278 | 1.080-4.803 | 56 | 2.016 | 1.037-3.916 | |||||
* Statistically significant
Survival analysis for UBUC
Follow-up information is available for all patients, with a median duration of 23.1 months (range, 1-109). As illustrated in Table 4, in multivariate analyses, pT stage, mitotic rate, and SPOCK1 expression were significantly associated with both inferior DSS (P<0.001, P=0.015 and P<0.001, respectively) and MeFS (P=0.008, P=0.009 and P<0.001, respectively). Perineurial invasion was also predictive of DSS.
Table 4.
Univariate log-rank and multivariate analyses for Disease-specific and Metastasis-free Survivals in urinary bladder urothelial carcinoma.
| Parameter | Category | Case No. | Disease-specific Survival | Metastasis-free Survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| No. of event | P value | R.R. | 95% C.I. | P value | No. of event | P value | R.R. | 95% C.I. | P value | |||
| Gender | Male | 216 | 41 | 0.4906 | - | - | - | 61 | 0.2745 | - | - | - |
| Female | 79 | 11 | - | - | - | 16 | - | - | - | |||
| Age (years) | < 65 | 121 | 17 | 0.1315 | - | - | - | 32 | 0.8786 | - | - | - |
| ≥ 65 | 174 | 35 | - | - | - | 45 | ||||||
| Primary tumor (T) |
Ta | 84 | 1 | <0.0001* | 1 | - | <0.001* | 4 | <0.0001* | 1 | - | 0.008* |
| T1 | 88 | 9 | 2.823 | 0.307-25.968 | 23 | 2.595 | 0.757-8.891 | |||||
| T2-T4 | 123 | 42 | 12.821 | 1.459-112.681 | 50 | 4.363 | 1.271-14.977 | |||||
| Nodal metastasis |
Negative (N0) | 266 | 41 | 0.0001* | 1 | - | 0.444 | 61 | <0.0001* | 1 | - | 0.009* |
| Positive (N1-N2) | 29 | 11 | 1.315 | 0.652-2.650 | 16 | 2.216 | 1.217-4.034 | |||||
| Histological grade | Low grade | 56 | 2 | 0.0016* | 1 | - | 0.899 | 5 | 0.0007* | 1 | - | 0.891 |
| High grade | 239 | 50 | 1.101 | 0.248-4.880 | 72 | 1.073 | 0.393-2.927 | |||||
| Vascular invasion |
Absent | 246 | 37 | 0.0010* | 1 | - | 0.032* | 54 | <0.0001* | 1 | - | 0.600 |
| Present | 49 | 15 | 2.096 | 1.064-4.132 | 23 | 1.167 | 0.654-2.083 | |||||
| Perineural invasion |
Absent | 275 | 44 | <0.0001* | 1 | - | 0.025* | 67 | 0.0003* | 1 | - | 0.161 |
| Present | 20 | 8 | 2.601 | 1.127-6.004 | 10 | 1.696 | 0.810-3.554 | |||||
| Mitotic rate (per 10 high power fields) | < 10 | 139 | 12 | 0.0001* | 1 | - | 0.015* | 23 | <0.0002* | 1 | - | 0.009* |
| >= 10 | 156 | 40 | 2.279 | 1.170-4.439 | 54 | 1.958 | 1.181-3.247 | |||||
| SPOCK1 expression |
Low | 147 | 5 | <0.0001* | 1 | - | <0.001* | 13 | <0.0001* | 1 | - | <0.001* |
| High | 148 | 47 | 6.965 | 2.725-17.801 | 64 | 4.675 | 2.515-8.690 | |||||
* Statistically significant
Prognostic significance of SPOCK1 expression in UC
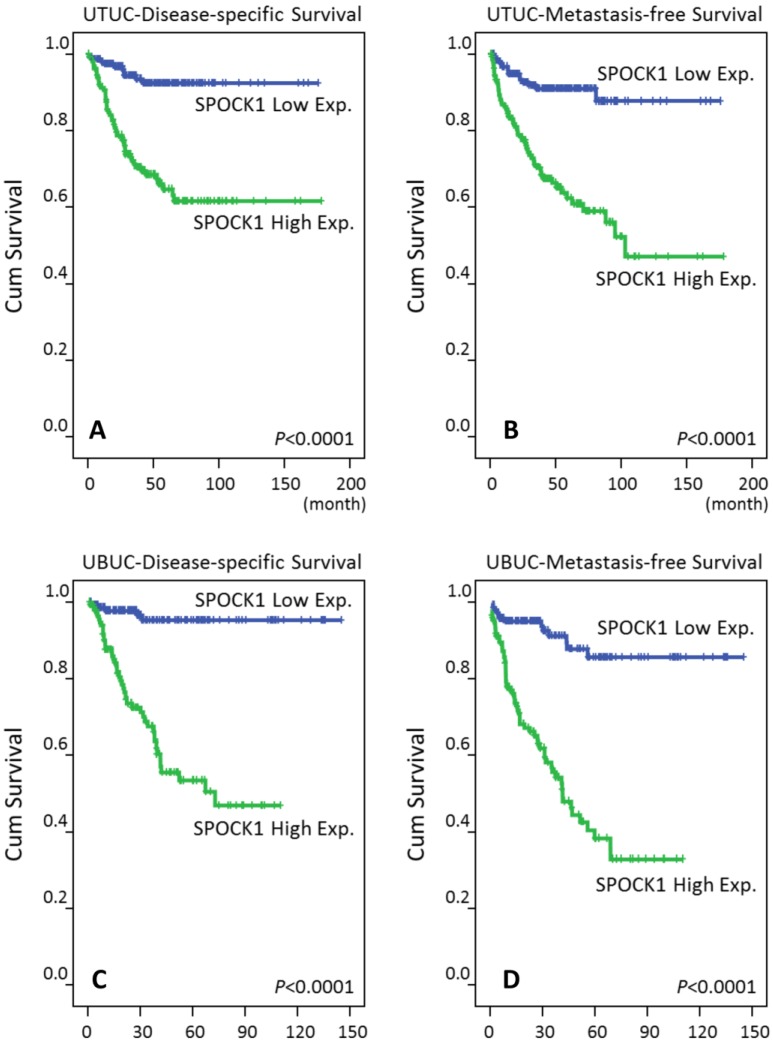
In the univariate analyses (Table 3), patients with UTUC showing high SPOCK1 expression had significantly worse DSS (P<0.0001, Fig. 4A) and MeFS (P<0.0001, Fig. 4B). Similar results were also noted for patients with UBUC (Table 4, Fig. 4C, and Fig. 4D). Of regard, SPOCK1 overexpression remained an independent prognosticator portending poor DSS and MeFS for both UTUC and UBUC patients (Table 3and Table 4).
Figure 4.
Survival analysis plotted by Kaplan-Meier curves. By log-rank test, high expression of SPOCK1 is predictive for inferior disease-free survival in both UTUC and UBUC (A, C) and for poor metastasis-free survival in both UTUC and UBUC (B, D).
Discussions
Interesting in markers of UC prognosis, the study identified five significantly expressed genes related to cell motility due to that invasion and metastasis had been suggested associating with adverse outcome in patients with UC. Among those genes, both CALD1 and SPOCK1 were expressed significantly, fitting with a log2 ratio over 1 and 0.5 fold in primary tumor status and distal metastasis, respectively (Table 1). CALD1 is involved in cell motility through the organization of the actin skeleton and actin/myosin-dependent contractility 14. SPOCK1, a highly conserved, multidomain proteoglycan in the extracellular matrix, could play a role in modulating protease activity 7. In the pilot study, only SPOCK1 exhibited crucial differences in primary tumor status, nodal metastasis and survival (Table S1, Table S2), implying its importance in UC aggressiveness compared to CALD1. In Fig. 2, higher pT was related to higher SPOCK1 mRNA expression. In addition, worse DSSs and MeFSs were associated with higher SPOCK1expression (Fig. 4). Aforementioned data may support the probable utility of SPOCK1 as a poor prognostic factor of UC.
SPOCK1, known as testican-1, was originally identified in seminal plasma 15. The expression of SPOCK1 mRNA in humans has been noted in various tissues with the highest levels in the brain, the prostate, and the testis 16. A study showed that a de novo missense change in SPOCK1 identified by whole exon sequencing might relate to developmental delay, microcephaly and agenesis of the corpus callosum, leading to the hypothesis that SPOCK1 plays a critical role in neurogenesis 17. SPOCK1 contains three domains viz., a follistatin domain, an extracellular calcium-binding domain, and a thyroglobulin type-1 domain, which possess functions of regulating metalloproteinase, and cysteine and serine proteases 16, 17. In a genome-wide association study of age at menarche, SPOCK1 was identified as a novel gene and arousing speculation about the underlying mechanism regarding its influence on matrix metalloproteinase-2 18.
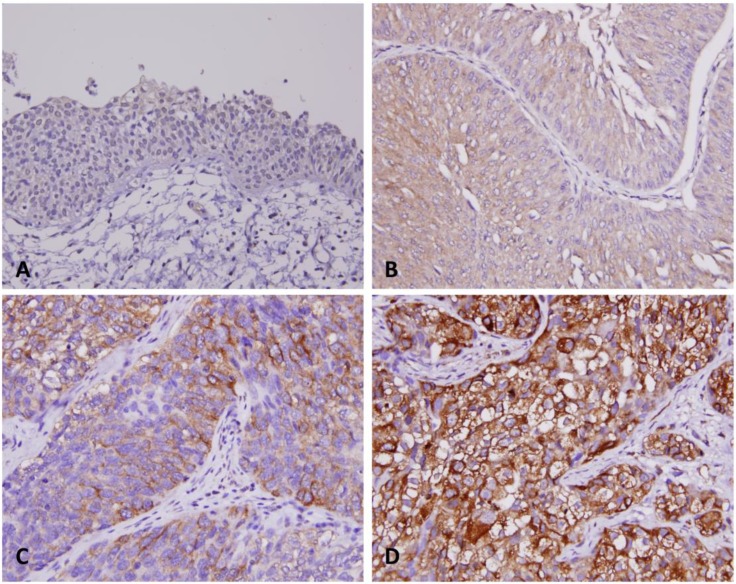
Furthermore, in the study of gastrointestinal neuroendocrine carcinomas, SPOCK1 was identified by microarray analysis as a candidate related to metastasis and was expressed 100-fold more in tumor material than in normal ileum 19. Upregulated SPOCK1 has also been identified in lung cancer 20, HCC 10, and gallbladder cancer 21. The clinical significance of SPOCK1 in lung cancer was correlated with metastasis and silencing of SPOCK1 in cell study inhibited lung cancer cell invasion in vitro 20. In addition, the tumorigenic ability of SPOCK1 in HCC was investigated in a xenograft mouse model with promising results 10. In our findings, SPOCK1 was also expressed more strongly in tumor tissues than in normal ones (Fig. 3). Moreover, higher expression of SPOCK1 is also related to positive nodal metastasis and presence of vascular and perineural invasion (Table 2), suggesting that it may have a role in UC invasion and metastasis, as noted with other cancers.
Figure 3.
Immunohistochemical staining for SPOCK1 in representative urothelium. Compared with surrounding non-tumorous tissues (A), the tumor tissues have significantly escalated immunostaining for SPOCK1 from non-invasive (B), superficially invasive (C) and deeply invasive urithelial carcinoma (D).
Also, altered composition of the extracellular matrix (ECM) is important in cancer. Epithelial-mesenchymal transition (EMT) involves changes in the cells themselves and in their surrounding microenvironment. Many studies support that obtaining epithelial-mesenchymal transition (EMT) features correlates with poor outcome among patients with various cancer types, including colorectal 22, lung 23, breast 24, and bladder cancer 25. SPOCK1, the ECM gene, was not only overexpressed in prostate cancer tissues compared to benign samples, but also indicated a tendency toward earlier recurrence 26. Interestingly, our study showed that high levels of SPOCK1 expression are correlated to poor DSS and MFS in UC in univariate log-rank tests (Table 3, Table 4). In vivo studies of lung cancer demonstrated that SPOCK1 is not only associated with metastasis and also induces EMT 20. Aforementioned facts may suggest that in UC, SPOCK1 may be a novel prognostic factor and may participate in tumor invasion and metastasis through involvement in the process of EMT.
Moreover, evading apoptosis is another capability that characterizes cancer cells 27. One study of GBC found that SPOCK1 not only promotes cell migration and invasion by inducing EMT but also inhibits apoptosis 21. The PI3 kinase-ATK/PKB pathway is important in affecting apoptosis 27. In a mouse model, this signal pathway was demonstrated to have role in upper tract of UC 28. The PI3 kinase-ATK/PKB pathway is also a downstream kinase pathway triggered by FGFR3, one of the important molecules involving bladder of UC 29. Furthermore, higher SPOCK1 expression was correlated with higher mitotic rate, as shown in Table-2, indicating that the adverse prognosis associated with high SPOCK1 expression may also relate to cell proliferation.
In conclusion, we present that higher SPOCK1 is related to unfavorable clinicopathological parameters and shorter survival in UC patients. Hypothetically, SPOCK1 may play an important role in the EMT of UC, and may be crucial in UC metastasis. Probably, SPOCK1 may also take a part in evading apoptosis through cell proliferation. This study suggested that SPOCK1 could be an independent prognostic factor in UC, might serve as an oncogene and would be a candidate of target therapy.
Supplementary Material
Tables S1-S2.
Acknowledgments
This study was supported by Chi Mei Medical Center (CMFHR10303), Ministry of Health and Welfare (MOHW103-TD-B-111-05) and E-Da Hospital (EDAHP104022). The authors are grateful to the BioBank of Chi Mei Medical Center to provide the tumor samples.
Ethical standard
This study was approved by the Institutional Review Board (IRB) of Chi Mei Medical Center, approval number IRB10302015. All samples were obtained from the BioBank of Chi Mei Medical Center and had been previously collected following official ethical guidelines. Informed consent has been obtained for those enrolled into BioBank.
Abbreviations
- DSS
disease-specific survival
- MeFS
metastasis-free survival
- SPOCK1
sparc/osteonectin, cwcv and kazal-like domains proteoglycan (testican) 1
- UBUC
urinary bladder urothelial carcinoma
- UC
urothelial carcinoma
- UTUC
upper tract urothelial carcinoma.
References
- 1.Liao AC, Li CF, Shen KH. et al. Loss of lactate dehydrogenase B subunit expression is correlated with tumour progression and independently predicts inferior disease-specific survival in urinary bladder urothelial carcinoma. Pathology. 2011;43(7):707–712. doi: 10.1097/PAT.0b013e32834bf67a. [DOI] [PubMed] [Google Scholar]
- 2.Liang PI, Li WM, Wang YH. et al. HuR cytoplasmic expression is associated with increased cyclin A expression and poor outcome with upper urinary tract urothelial carcinoma. BMC Cancer. 2012;12:611.. doi: 10.1186/1471-2407-12-611. doi: 10.1186/1471-2407-12-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng L, Zhang S, MacLennan GT. et al. Bladder cancer: translating molecular genetic insights into clinical practice. Hum Pathol. 2011;42(4):455–481. doi: 10.1016/j.humpath.2010.07.007. doi: 10.1016/j.humpath.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Cheng L, Lopez-Beltran A, MacLennan GT, et al. Urologic surgical pathology, 2nd ed. Philadelphia: Elsevier/Mosby; 2008. Neoplasms of the urinary bladder; pp. 259–352. [Google Scholar]
- 5.Fujita K, Uemura M, Yamamoto Y. et al. Preoperative risk stratification for cancer-specific survival of patients with upper urinary tract urothelial carcinoma treated by nephroureterectomy. Int J Clin Oncol. 2015;20(1):156–163. doi: 10.1007/s10147-014-0695-1. doi: 10.1007/s10147-014-0695-1. [DOI] [PubMed] [Google Scholar]
- 6.UpToDate: Joaquim Bellmunt. Treatment of metastatic urothelial cancer of the bladder and urinary tract. This topic last updated: June 26, 2015. http://www.uptodate.com/contents/treatment-of-metastatic-urothelial-cancer-of-the-bladder-and-urinary-tract?source=search_result&search=treatment+of+metastatic+urothelial&selectedTitle=1%7E150.
- 7.Bradshaw AD. Diverse biological functions of the SPARC family of proteins. Int J Biochem Cell Biol. 2012;44(3):480–488. doi: 10.1016/j.biocel.2011.12.021. doi: 10.1016/j.biocel.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tai IT, Tang MJ. SPARC in cancer biology: its role in cancer progression and potential for therapy. Drug Resist Update. 2008;11(6):231–246. doi: 10.1016/j.drup.2008.08.005. doi: 10.1016/j.drup.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Colin C, Baeza N, Bartoli C. et al. Identification of genes differentially expressed in glioblastoma versus pilocytic astrocytoma using suppression subtractive hybridization. Oncogene. 2006;25(19):2818–2826. doi: 10.1038/sj.onc.1209305. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Chen L, Chan TH. et al. SPOCK1 is regulated by CHD1L and blocks apoptosis and promotes HCC cell invasiveness and metastasis in mice. Gastroenterology. 2013;144(1):179–191. doi: 10.1053/j.gastro.2012.09.042. e4. doi: 10.1053/j.gastro.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 11.Liang PI, Wang YH, Wu TF. et al. IGFBP-5 overexpression as a poor prognostic factor in patients with urothelial carcinomas of upper urinary tracts and urinary bladder. J Clin Pathol. 2013;66(7):573–582. doi: 10.1136/jclinpath-2012-201278. doi: 10.1136/jclinpath-2012-201278. [DOI] [PubMed] [Google Scholar]
- 12.Budwit-Novotny DA, McCarty KS, Cox EB. et al. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46(10):5419–5425. [PubMed] [Google Scholar]
- 13.McClelland RA, Finlay P, Walker KJ. et al. Automated quantitation of immunocytochemically localized estrogen receptors in human breast cancer. Cancer Res. 1990;50(12):3545–3550. [PubMed] [Google Scholar]
- 14.Mayanagi T, Sobue K. Diversification of caldesmon-linked actin cytoskeleton in cell motility. Cell Adh Migr. 2011;5(2):150–159. doi: 10.4161/cam.5.2.14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alliel PM, Perin JP, Jollès P. et al. Testican, a multidomain testicular proteoglycan resembling modulators of cell social behaviour. Eur J Biochem. 1993;214(1):347–350. doi: 10.1111/j.1432-1033.1993.tb17930.x. [DOI] [PubMed] [Google Scholar]
- 16.Edgell CJ, BaSalamah MA, Marr HS. Testican-1: a differentially expressed proteoglycan with protease inhibiting activities. Int Rev Cytol. 2004;236:101–122. doi: 10.1016/S0074-7696(04)36003-1. [DOI] [PubMed] [Google Scholar]
- 17.Dhamija R, Graham JM Jr, Smaoui N. et al. Novel de novo SPOCK1 mutation in a proband with developmental delay, microcephaly and agenesis of corpus callosum. Eur J Med Genet. 2014;57(4):181–184. doi: 10.1016/j.ejmg.2014.02.009. doi: 10.1016/j.ejmg.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Liu YZ, Guo YF, Wang L. et al. Genome-wide association analyses identify SPOCK as a key novel gene underlying age at menarche. PLoS Genet. 2009;5(3):e1000420.. doi: 10.1371/journal.pgen.1000420. doi: 10.1371/journal.pgen.1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leja J, Essaghir A, Essand M. et al. Novel markers for enterochromaffin cells and gastrointestinal neuroendocrine carcinomas. Mod Pathol. 2009;22(2):261–272. doi: 10.1038/modpathol.2008.174. doi: 10.1038/modpathol.2008.174. [DOI] [PubMed] [Google Scholar]
- 20.Miao L, Wang Y, Xia H. et al. SPOCK1 is a novel transforming growth factor-β target gene that regulates lung cancer cell epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2013;440(4):792–797. doi: 10.1016/j.bbrc.2013.10.024. doi: 10.1016/j.bbrc.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Shu YJ, Weng H, Ye YY. et al. SPOCK1 as a potential cancer prognostic marker promotes the proliferation and metastasis of gallbladder cancer cells by activating the PI3K/AKT pathway. Mol Cancer. 2015;14:12.. doi: 10.1186/s12943-014-0276-y. doi: 10.1186/s12943-014-0276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shan ZZ, Yan XB, Yan LL. et al. Overexpression of Tbx3 is correlated with epithelial-mesenchymal transition phenotype and predicts poor prognosis of colorectal cancer. Am J Cancer Res. 2014;5(1):344–353. [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Guan X, Zhang H. et al. DAL-1 attenuates epithelial to mesenchymal transition in lung cancer. J Exp Clin Cancer Res. 2015;34:3.. doi: 10.1186/s13046-014-0117-2. doi: 10.1186/s13046-014-0117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro AS, Paredes J. P-Cadherin linking breast cancer stem cells and invasion: a promising marker to identify an “intermediates/metastable” EMT state. Front Oncol. 2015;4:371.. doi: 10.3389/fonc.2014.00371. doi: 10.3389/fonc.2014.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adam L, Zhong M, Choi W. et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res. 2009;15(16):5060–5072. doi: 10.1158/1078-0432.CCR-08-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wlazlinski A, Engers R, Hoffmann MJ. et al. Downregulation of several fibulin genes in prostate cancer. Prostate. 2007;67(16):1770–1780. doi: 10.1002/pros.20667. [DOI] [PubMed] [Google Scholar]
- 27.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 28.Qian CN, Furge KA, Knol J. et al. Activation of the PI3K/AKT pathway induces urothelial carcinoma of the renal pelvis: identification in human tumors and confirmation in animal models. Cancer Res. 2009;69(21):8256–8264. doi: 10.1158/0008-5472.CAN-09-1689. doi: 10.1158/0008-5472.CAN-09-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng L, Zhang S, MacLennan GT. et al. Bladder cancer: translating molecular genetic insights into clinical practice. Hum Pathol. 2011;42(4):455–481. doi: 10.1016/j.humpath.2010.07.007. doi: 10.1016/j.humpath.2010.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1-S2.