Summary
Tandem repetitive DNA is highly abundant in eukaryotic genomes, and contributes to transcription control and genome stability. However, how the individual sequences within tandem repeats behave remains largely unknown. Here we develop a collection of fission yeast strains with a reporter gene inserted at different units in a tandem repeat array. We show that, contrary to what is usually assumed, transcriptional silencing and replication timing among the individual repeats differ significantly. RNAi-mediated H3K9 methylation is essential for the silencing position effect. A short hairpin RNA of ura4+ induces silencing in trans within the tandem array in a position-dependent manner. Importantly, the position effect depends on the condensin subunit, cut3+. Cut3 promotes the position effect via interaction with the RNA-induced transcriptional silencing (RITS) complex. This study reveals variations in silencing within tandem DNA repeats and provides mechanistic insights into how DNA repeats at the individual level are regulated.
Introduction
In eukaryotic cells, tandem repetitive DNA sequences occupy a substantial fraction of the genome. For example, tandem repetitive DNA arrays make up about 10% of mammalian genomes (Richard et al., 2008; Warburton et al., 2008). Initially considered “junk” DNA, repetitive DNA sequences now have been implicated in transcription control, genome stability and development (Martienssen et al., 2004; Richard et al., 2008; Shapiro and von Sternberg, 2005). However, due to their repetitive nature, it is difficult to study tandem DNA sequences. As a result, tandem repeat arrays remain among the most poorly understood structures in the genome.
DNA repeats often are organized into heterochromatin, the highly condensed and transcriptionally silenced chromatin domain (Martienssen et al., 2004). Heterochromatin structure and function have been extensively studied. Yet, very little is known about how the heterochromatin at the individual repeat level is regulated. It has been assumed that the silenced heterochromatin state is uniformly distributed within a tandem repeat array.
In many eukaryotes, including humans, peri-centromeres are heterochromatic and consist of long arrays of tandem repetitive DNA (Allshire and Karpen, 2008; Martienssen et al., 2004). The peri-centromeric heterochromatin has been linked to centromere function and chromosome segregation (Boyarchuk et al., 2014; Folco et al., 2008; Gonzalez et al., 2014; Smith et al., 2011).
Like many other eukaryotes, peri-centromeres in fission yeast (Schizosaccharomyces pombe) comprise arrays of large heterochromatic DNA repeats that have been used widely to study heterochromatin. Fission yeast has 3 chromosomes, each containing a single centromere, ranging from 35 kb to 110 kb (Wood et al., 2002). The core region of the centromeres (cnt, centromere core domain) is enriched with the CENP-A homolog Cnp1 (CENP-Acnp1), a centromeric specific histone H3 variant that defines centromere identity (Allshire and Karpen, 2008; Takahashi et al., 2000). Immediately flanking cnt are the innermost repeat regions (imr), which include imperfect inverted repeat elements. Outside of the imr region are the outmost repeat regions (otr). The otr regions contain large repeats, each of which consists of dg and dh elements and spans approximately 6.7 kb (Wood et al., 2002). Both imr and otr regions are heterochromatic (Allshire et al., 1994), and are enriched for methylation at histone H3 lysine 9 (H3K9me), the conserved epigenetic mark of heterochromatin. H3K9me is regulated by the ClrC complex, which contains Clr4, the H3K9 methyltransferase Rik1, Cul4, Dos1, and Dos2 (Hong et al., 2005; Horn et al., 2005; Jia et al., 2005; Li et al., 2005; Thon et al., 2005). RNA interference (RNAi) is also required for H3K9me and heterochromatin silencing. Fission yeast contains a single copy of Argonaute (Ago1), Dicer (Dcr1), and the RNA dependent RNA polymerase (Rdp1). Ago1, together with the chromo-domain proteins Chp1 and Tas3, is assembled into the RITS (RNA-induced transcriptional silencing) complex (Motamedi et al., 2004; Volpe et al., 2002). During S phase of the cell cycle, the DNA Pol epsilon subunit Cdc20 promotes the transcription of peri-centromeric heterochromatin. RNAi subsequently processes the peri-centromeric transcripts into siRNAs, which in turn facilitate recruitment of the ClrC complex to the heterochromatin region (Chen et al., 2008; Kloc et al., 2008; Li et al., 2011a; Zaratiegui et al., 2011).
To directly probe the behavior of individual repeats in tandem repeat arrays, we developed a collection of strains carrying a reporter gene in different otr repeats. Our results demonstrate that heterochromatin silencing and replication timing between different otr repeats can vary significantly. The position effect is dependent on RNAi and Cut3, a subunit of the condensin complex. Cut3 mutation also results in mislocalization of CENP-Acnp1. This study reveals previously unknown position effects within tandem DNA repeats, and suggests a mechanism for how DNA repeats at the individual level are regulated. Our study also implicates the position effects at peri-centromeric regions as a possible contributor in the CENP-A positioning at centromere.
Results
Construction of strains for analyzing silencing in individual otr repeats
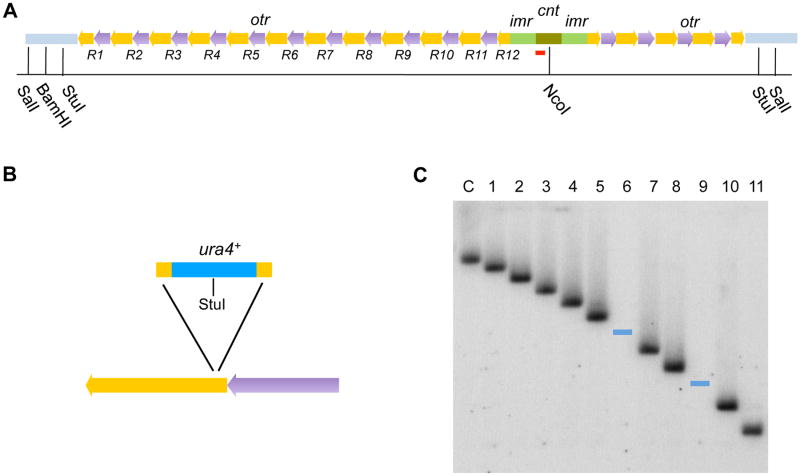
To examine the level of silencing in each individual DNA repeat within the otr tandem array of chromosome 3 (Chr3), we created a collection of strains, in which the ura4+ reporter was inserted in a single otr repeat in each strain (Figure 1A). The otr region in Chr3 has the largest number of repeats among the three centromeres. The otr region at the right side of the Chr3 centromere contains four full-length 6.7kb repeats. The number of otr repeats at the left side (otr3L), is not well defined, with estimated numbers ranging from 7 to 11 (Ellermeier et al., 2010; Wood et al., 2002). However, it is known that the first and last of the otr3L repeats contain only partial otr sequence (Wood et al., 2002).
Figure 1. Construction of Strains Carrying a ura4+ Reporter Inserted at Different Repeat Units Within the otr Tandem Array.
(A) Schematic structure of centromeric core (cnt) and peri-centromeric DNA repeats in fission yeast Chr3. Each outer repeat (otr) contains a dg (yellow) and dh (purple) element. Red lines indicate the position of probes used for the Southern blot analysis.
(B) Schematic diagram of insertion of a ura4+ gene into a single otr repeat.
(C) Southern blot analysis of transformants carrying ura4+ at individual otr3L repeats. DNA of the transformants was digested with NcoI and StuI, and probed for the core centromeric region. ura4+ was successfully inserted at each repeat in otr3L region except repeats 6, 9 and 12. Blue lines mark the position of the predicted bands for digestion of DNA from cells carrying ura4+ in repeat 6 or 9 of the otr3L region. C, control strain without ura4+ at the otr region.
Using a recently described two-step process (Vader et al., 2011), we successfully obtained strains carrying ura4+ reporter inserted at each repeat in otr3L region except repeats 6, 9 and 12 (Figures 1B and 1C). Based on this analysis, we concluded that the left arm of Chr3 contains a total of twelve otr repeats, including the first and last incomplete repeats (Figure 1C). Further Southern blotting analysis indicated that, in a portion of the transformants, the total length of the otr3L array was different from the size of the otr region in WT (Figure S1), likely resulting from aberrant, non-allelic recombination. In this study, we only focused on the transformants carrying the wild-type length of the otr region.
otr tandem repeats exhibit position-dependent silencing
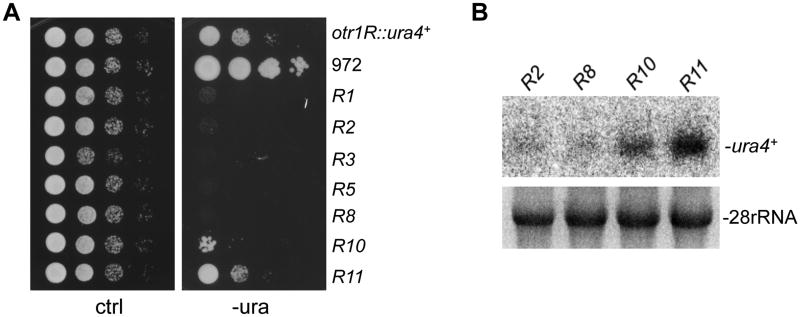
To evaluate the level of silencing at each individual otr repeat, strains from our collection were analyzed by growth assays on medium lacking uracil (-ura). The growth rate of these strains differs on -ura medium (Figure 2A), indicating that different otr repeats exhibit variation in heterochromatin silencing. We found that cells carrying the ura4+ reporter inserted in the repeats distal to the centromere core, including repeats 2-8, have the slowest growth on -ura medium, indicating that these repeats are strongly silenced. Interestingly, repeat 1, which contains a partial otr repeat, also displays strong silencing. On the other hand, silencing of repeats close to the centromere is substantially reduced. Repeat 11, the rightmost repeat carrying the reporter, appears to exhibit the weakest silencing (Figure 2A). The position-dependent silencing within the otr region was further confirmed by Northern blotting (Figures 2B and S2A). Our results thus demonstrate that silencing in the centromeric otr repeats is profoundly position-dependent.
Figure 2. Silencing Level in otr Tandem Repeats is Position-Dependent.
(A) Serial dilutions of cells harboring ura4+ inserted in the indicated otr3L repeats were plated on minimal medium without uracil (-ura). Strain 972, wild type strain harboring ura4+ at the endogenous locus. otr1R∷ura4+, wild-type control strain carrying a ura4+ at the otr region in Chr1 (Allshire et al., 1995).
(B) Total RNA was prepared from cells carrying ura4+ inserted in the indicated otr3L repeats, and analyzed by Northern blot assay with a probe specific for ura4+. 28S rRNA was used as a loading control.
To assess whether the silencing state of individual repeats can be inherited through generations, we backcrossed the strains carrying ura4+ in otr repeat 2, 8 or 11 multiple times to a parental strain lacking the reporter. Our growth assays indicate that the specific silencing state in different otr repeats is stably inherited (Figures S2B and S2C).
H3K9 methylation and RNAi are essential for the position effect in otr repeats
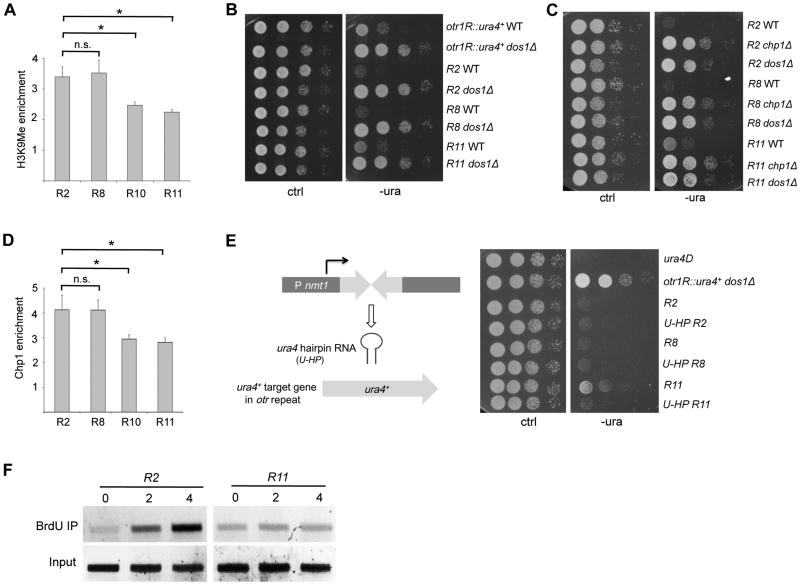
H3K9me plays an important role in heterochromatin silencing (Nakayama et al., 2001). Our chromatin immunoprecipitation (ChIP) assays showed that H3K9me was detected in all repeats examined; however, repeats 2 and 8 contained relatively higher level of H3K9me than repeats 10 and 11 (Figure 3A). These results indicate that the H3K9me and chromatin silencing level in the repeats are highly correlated. Furthermore, deletion of Dos1, a key component of the ClrC complex (Li et al., 2005), abolishes the position effect in the otr repeats (Figure 3B), indicating that the position-dependent regulation of heterochromatic silencing at the otr repeats acts upstream of H3K9me.
Figure 3. Position Effect in otr Repeats Depends on H3K9 Methylation and RNAi.
(A) Analysis of H3K9 methylation in repeats 2, 8, 10, or 11 in the otr region by ChIP. Wild type cells carrying ura4+ in the indicated- repeats were used. ChIP assays were performed using an antibody against H3K9me and primers specific for ura4+. act1+ was used as a control. Three independent experiments were performed. Error bars indicate SD. (*) P < 0.05. n.s., not significant.
(B) Serial dilutions of dos1Δ mutant cells with ura4+ in otr3L repeats 2, 8 or 11 were spotted on –ura medium, and incubated at 25°C.
(C) Growth assay for dcr1Δ mutant carrying ura4+ in otr3L repeats 2, 8 or 11. Serial dilution of dcr1Δ strains was plated on –ura medium.
(D) ChIP analysis of Chp1-GFP in otr repeats 2, 8, 10, or 11. Wild type cells carrying Chp1-GFP and the ura4+ in indicated repeats were used. Error bars indicate SD.
(E) A hairpin of ura4+ (U-HP) (Simmer et al., 2010) induces silencing in otr repeats in trans in a position-dependent manner. Cells carrying the ura4+ hairpin and the ura4+ reporter in the indicated repeats were analyzed by growth assays in -ura medium.
(F) Cells carrying ura4+ in otr3L repeat 2 or 11 were analyzed by BrdU-IP. Cells collected at the indicated time points (hrs).
Assembly of peri-centromeric heterochromatin requires the RNAi machinery (Volpe et al., 2002). Our growth assays showed that, similar to the dos1Δ background, position-dependent silencing in dcr1Δ strains with ura4+ in different repeats is eliminated (Figure S3A). We also observed a similar loss of position effect in the deletion mutant of Chp1, a component of the RITS complex (Figure 3C). Furthermore, our ChIP analysis demonstrated that Chp1 is highly enriched in repeats 2 and 8, but decreased in repeats 10 and 11 (Figure 3D), a pattern consistent with the silencing state in these individual repeats.
A ura4+ hairpin induces silencing within otr repeats in trans in a position-dependent manner
A hairpin structure of ura4+ in fission yeast can induce heterochromatin silencing in trans at a target locus near heterochromatin, but has only minor effects on silencing within the single otr repeat in Chr1 (Iida et al., 2008; Simmer et al., 2010). To determine whether heterochromatin silencing can be induced by the ura4+ hairpin in trans in the otr tandem repeats, we used a hairpin, which contains a sequence complementary to 200bp of ura4+ under the nmt1 promoter (U-HP), and was integrated on chromosome 1 (Simmer et al., 2010). We found that expression of U-HP can induce strong silencing in trans in repeat 11, but has little effect on silencing in otr repeat 2 or 8 (Figure 3E). We also found that expression of U-HP in the dcr1Δ mutant cells did not result in silencing in the otr repeats, indicating that the hairpin-mediated trans-silencing requires RNAi (Figure S3B). Together, our result support the idea that siRNAs generated by the ura4+ hairpin induce silencing in otr repeats in trans, and that this construct can overcome the position-dependent regulation of heterochromatic silencing in the otr repeats. The weak effect of ura4+ hairpin expression on silencing in repeats 2 and 8 may be due to the fact that these regions already form a highly condensed heterochromatin structure.
Replication timing varies between different otr repeats
DNA replication contributes to pericentromeric heterochromatin formation in fission yeast (Li et al., 2011a). Our collection of repeat-specific reporters provides us the opportunity to analyze how replication timing differs between individual repeats. Using BrdU-IP, we observed that the incorporation of BrdU into repeats 10 and 11 is severely delayed compared to its incorporation into repeat 2 using the BrdU-ChIP (Figures 3F and S3C). These results indicate that the strongly silenced repeats in otr region (i.e. repeat 2) replicate earlier than the weakly silenced repeats 10 and 11.
Cut3, a condensin subunit, promotes the position effect in otr repeats via the RITS complex
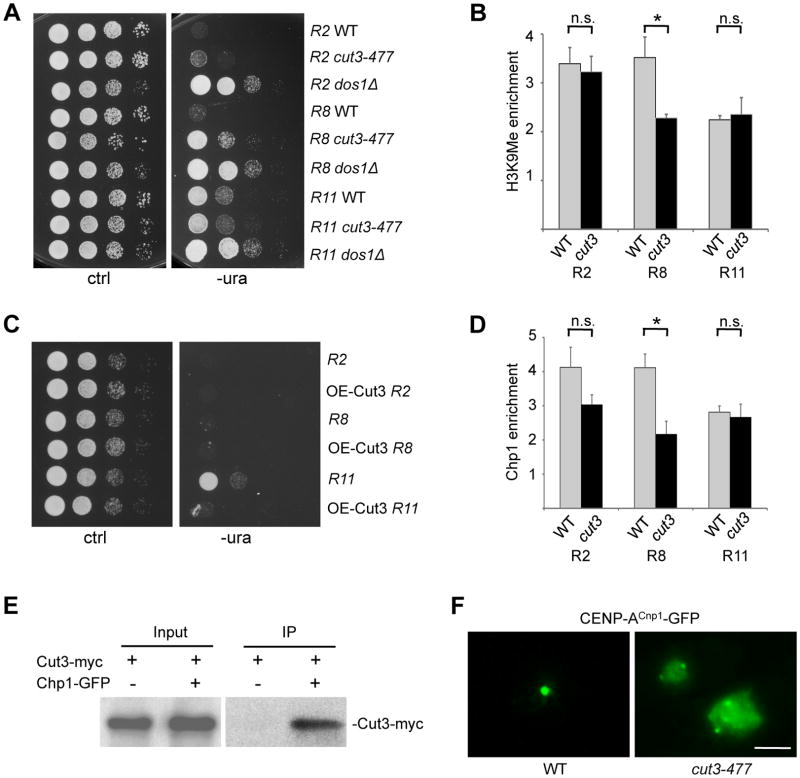
Condensin, a protein complex that is essential for chromosome condensation during mitosis (Hudson et al., 2009), has been implicated in heterochromatin function (Chen et al., 2008; Oliveira et al., 2005). To determine whether condensin is involved in the position effect on silencing in the otr repeats, we analyzed the silencing states in otr repeats in a condensin mutant, cut3-477. cut3-477 is a temperature sensitive mutant that is unable to grow at 37°C (Saka et al., 1994). We found that the position-dependent silencing pattern across the otr region is severely disrupted in cut3-477 mutant even at 32°C. However, unlike dos1Δ and RNAi mutants, which exhibit a total loss of silencing in all repeats tested, the effect of the cut3-477 mutation on silencing varied between repeats. While silencing is only mildly reduced in the mutant in repeat 2, silencing in repeat 8 is significantly lost. In contrast, the mutation has little effect on the silencing in repeat 11 (Figure 4A). Consistent with this, H3K9me is drastically reduced in repeat 8 in cut3-477 mutant (Figure 4B). We also observed that heterochromatin silencing is substantially decreased in repeat 1 and 5 (Figure S4A). Furthermore, we found that overexpression of Cut3 can enhance silencing in the repeat 11, but has no obvious effect on silencing in the repeats 2 and 8 (Figure 4C). These results indicate that Cut3 is a key regulator of the position effect in otr repeats. However, we found that the association of Cut3 with repeats 2, 8, and 11 is not significantly different (Figure S4B). In addition, disruption of centromere structure using a mutant of Mis6, an essential centromere protein, results in no obvious effect on Cut3 distribution between different repeats (Figure S4C). These data – suggest that additional factor may be required for the position effect.
Figure 4. Condensin Regulates the Position Effect Within otr Repeats via RITS and is Essential for CENP-A Centromeric Localization.
(A) cut3-477 mutant cells with ura4+ in otr repeats 2, 8 or 11 were serially diluted and spotted on the –ura medium, and incubated at 32°C.
(B) ChIP analysis of H3K9 methylation in otr repeats 2, 8 and 11 in cut3-477. Error bars indicate SD. (*) P < 0.05. n.s., not significant.
(C) Overexpression of Cut3 perturbs the position-dependent silencing in otr repeats. Serial dilution of wild--type cells carrying ura4+ in indicated otr repeat and nmt1-cut3+ were incubated in –ura medium lacking thiamine. OE, overexpression.
(D) ChIP analysis of Chp1-GFP in otr3L repeats 2, 8 or 11 in cut3-477. Error bars indicate SD. (*) P < 0.05. n.s., not significant.
(E) Cut3-myc is co-immunoprecipitated with Chp1-GFP. Extracts from cells carrying Cut3-myc and Chp1-GFP was immunoprecipitated with a GFP antibody. Input and immunoprecipitate were analyzed by western blotting using the antibody against the myc tag.
(F) CENP-Acnp1 is mislocalized in cut3-477 mutant. cut3-477 cells expressing CENP-Acnp1-GFP at endogenous level were incubated at 34°C for 5 hrs. Scale bar, 2 μm.
To determine how Cut3 contributes to the position-dependent silencing in the otr repeats, we investigated how the recruitment of the RITS complex to different otr repeats is affected in cut3-477 mutants by ChIP. We found that the level of Chp1 in repeat 8 is greatly reduced in the mutant, whereas its level in repeat 2 and 11 is only mildly affected (Figure 4D), indicating that Cut3 contributes to the position-dependent recruitment of RITS. Furthermore, our co-IP assays showed that Cut3-myc is co-immunoprecipitated with Chp1-GFP (Figure 4E), demonstrating that Cut3 interacts with the RITS complex.
CENP-Acnp1 is delocalized in cut3-477
The core centromere is enriched for CENP-Acnp1 (Allshire and Karpen, 2008; Takahashi et al., 2000). To investigate whether the position effect at the pericentromeric otr repeats may play a role in the positioning of CENP-Acnp1, we examined CENP-Acnp1-GFP in cut3-477 cells. In wild-type S. pombe cells, centromeres are clustered together at the nuclear envelope. Thus, a single fluorescent focus is observed in most cells expressing CENP-Acnp1-GFP at endogenous level (Gonzalez et al., 2014). However, we found that 36% of cut3-477 cells incubated at 34°C for 5 hours exhibits multiple foci or diffused CENP-Acnp1-GFP pattern (Figure 4F), indicating that association of CENP-A with centromeres is severely perturbed in the mutant. Western blotting demonstrated that the level of CENP-Acnp1-GFP is not affected in cut3-477 cells (Figure S4D).
Discussion
Despite the importance of tandem DNA repeats (Martienssen et al., 2004; Shapiro and von Sternberg, 2005), how the individual sequences within the tandem repeats behave and how their function is regulated remain poorly understood. Using a collection of repeat-specific reporters, Vader et al. have shown that the edges of repetitive ribosomal DNA (rDNA) array in budding yeast are more susceptible to homologous recombination during meiosis (Vader et al., 2011). Here, using a similar approach, we demonstrated that transcriptional silencing in the different repeat units within the peri-centromeric tandem otr repeats can be strikingly different. An accompanying study by Wang et al. also revealed a transcriptional position effect in the repetitive rDNA regions in budding yeast, suggesting that position effects in tandem repeat arrays are conserved.
We show that the otr tandem repeats in fission yeast Chr3 exhibit striking position-dependent silencing. These findings suggest that, although different repeats in the tandem array share the same sequence, each is organized into a specific higher-order structure. We demonstrated that H3K9me and RNAi components are key effectors of the observed positional differences. Moreover, we found that condensin is essential for the formation of the position-dependent epigenetic states. In particular, the specific pattern of the RITS complex associated with different individual otr repeats is perturbed in the cut3 mutant. These results suggest that condensin acts as the upstream instructor to recruit the proper level of silencing effectors, including the RITS complex, to individual repeats, and establish the unique heterochromatin state in the particular position. Nevertheless, we did not observe any significant difference in the level of Cut3 associated with different peri-centromeric repeats, suggesting that additional factor(s) may be required to function together with Cut3 to promote the position effect.
What is the biological relevance of the position effect at the pericentromeric repeats? Centromeres are responsible for kinetochores assembly, and play a key role in chromosome segregation (Allshire and Karpen, 2008). In most other eukaryotes, centromere identity is believed to be predominantly epigenetically specified (Fukagawa and Earnshaw, 2014). CENP-A is the most likely candidate for the epigenetic mark used to define the centromeres. How CENP-A is incorporated to centromeric regions remains poorly understood, but mis-regulation of CENP-A adversely affects chromosome segregation, resulting in aneuploidy and cancer (Allshire and Karpen, 2008). Our studies suggest that the distinct three-dimensional architecture created by the tandem arrays at pericentromeres may contribute to proper positioning of CENP-A. Indeed, RNAi components and Clr4 have been shown to be required for establishment of CENP-Acnp1 chromatin at centromere in fission yeast (Folco et al., 2008; Gonzalez et al., 2014). Previous studies also demonstrate that peri-centromeric heterochromatin is important for centromeric localization of CENP-A in Neurospora crassa and mouse cell lines (Boyarchuk et al., 2014; Smith et al., 2011). Here, we show that the key regulator of the position effect in pericentromeric repeats, Cut3, is also required for the centromeric localization of CENP-Acnp1 (Figure 4F). Condensin has been previously implicated in assembly of CENP-A chromatin in Xenopus and human cells (Bernad et al., 2011; Samoshkin et al., 2009), but its precise role in the process is still unclear. We propose that condensin mediates the organization of peri-centromeric repeats into a specific higher-order structure, which in turn helps restrict CENP-A to centromeres.
We demonstrated that replication timing among the individual units in a tandem array can vary significantly. Replication of an otr repeat near the centromere is severely delayed compared to a more strongly silenced repeats close to the chromosome arm. Although heterochromatin in general is associated with late replication, centromeric heterochromatin in fission yeast and a few of other organisms replicates early in the S phase (Kim et al., 2003). The reason for the early replication of these heterochromatic regions remains unclear. During DNA replication, CENP-A chromatin is disassembled, and CENP-A must be faithfully reincorporated into centromeres after replication (Allshire and Karpen, 2008; Gonzalez et al., 2013). It has been shown that DNA replication is important for heterochromatin assembly at peri-centromeric regions in fission yeast (Chen et al., 2008; Kloc et al., 2008; Li et al., 2011a; Zaratiegui et al., 2011). We propose that early replication of strongly silenced peri-centromeric repeats allows the establishment of the position effect at an early stage so as to create a chromatin environment that can ensure the proper positioning of CENP-A. Consistent with this idea, the core region of centromeres, cnt, and the flanking imr regions, both of which are weakly silenced, replicate later than the highly heterochromatic otr regions (Li et al., 2011b).
Our findings reveal previously unrecognized position effects within tandem repeat arrays, and support the concept that the position effects at peri-centromeric repeats promote the epigenetic specification of centromeres. In addition, our studies uncover condensin and RNAi components as key factors involved in position-dependent epigenetic regulation of tandem DNA repeats, and provide insight into how DNA repeats at an individual level are controlled. It will be important in future studies to identify other key regulator of the position effects. It will also be interesting to explore how the tandem repeats are spatially organized. These studies will shed light on regulation of the important but poorly studied part of the genome.
Experimental Procedures
Strains, Media and Genetic analysis
Fission yeast strains used in this study are listed in Table S1. Standard media and genetic analysis for fission yeast were used (Moreno et al., 1991).
Strain Construction
The repeat-specific reporters were created using a recently described process with minor modifications (Vader et al., 2011), and further details are available in the Supplemental Information.
CHEF gel electrophoresis and Southern Blot
CHEF gel electrophoresis and Southern Blotting were performed as described (Vader et al., 2011) with minor modifications, and further details are available in the Supplemental Information.
Co-immunoprecipitation and Western Blot Analysis
Immunoprecipitation was performed using an anti-GFP antibody (Abcam, ab290). Eluates were analyzed by standard western blotting protocols using an anti-myc antibody (Sigma, C3956). For western blot analysis of the cut3 mutant, blots were probed with anti-GFP (Roche, 11 814 460 001) or α-tubulin (Abcam, ab6160) antibodies.
ChIP
ChIP was performed by as described (Li et al., 2008). Primers used are listed in Table S2. Quantifications were performed using ImageJ 1.46r software. All experiments were independently repeated three times. Two-tailed Student's t-test was used to determine the statistical significance between different experimental groups.
BrdU IP
BrdU IP was essentially performed as described previously (Li et al., 2011b). Primers used in this study are listed in Table S2.
Northern Blot
Northern blot was performed according to the standard protocol. Briefly, RNA samples were separated and transferred to an Amersham Hybond-N+ membrane in 2× SSC buffer. After UV cross-linking, the membranes were hybridized by 32P-labeled probe recognizing ura4+ or the 28S rRNA as a control, and exposed to film for autoradiography.
Supplementary Material
Acknowledgments
We are grateful to Robin Allshire, Mitsuhiro Yanagida, and The Japan Yeast Genetic Resource Center for kindly providing strains. We thank members of Li laboratory, particularly Marlyn Gonzalez, for critical reading of the manuscript. F. L. is a Pew Scholar in the Biomedical Sciences, supported by The Pew Charitable Trusts. This project was supported by NIH grant 1R01 GM106037 (to F.L.) and NSF grant MCB-1330557 (to F.L.).
Footnotes
Author Contributions: H.H. performed the experiments with assistance from S.Z. and D.W.; F.L. designed the study and wrote the manuscript with input from A.H.
Supplemental Information: Supporting Information includes four figures and two tables.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allshire RC, Javerzat JP, Redhead NJ, Cranston G. Position effect variegation at fission yeast centromeres. Cell. 1994;76:157–169. doi: 10.1016/0092-8674(94)90180-5. [DOI] [PubMed] [Google Scholar]
- Allshire RC, Karpen GH. Epigenetic regulation of centromeric chromatin: old dogs, new tricks? Nat Rev Genet. 2008;9:923–937. doi: 10.1038/nrg2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allshire RC, Nimmo ER, Ekwall K, Javerzat JP, Cranston G. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 1995;9:218–233. doi: 10.1101/gad.9.2.218. [DOI] [PubMed] [Google Scholar]
- Bernad R, Sanchez P, Rivera T, Rodriguez-Corsino M, Boyarchuk E, Vassias I, Ray-Gallet D, Arnaoutov A, Dasso M, Almouzni G, et al. Xenopus HJURP and condensin II are required for CENP-A assembly. J Cell Biol. 2011;192:569–582. doi: 10.1083/jcb.201005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyarchuk E, Filipescu D, Vassias I, Cantaloube S, Almouzni G. The histone variant composition of centromeres is controlled by the pericentric heterochromatin state during the cell cycle. J Cell Sci. 2014;127:3347–3359. doi: 10.1242/jcs.148189. [DOI] [PubMed] [Google Scholar]
- Chen ES, Zhang K, Nicolas E, Cam HP, Zofall M, Grewal SIS. Cell cycle control of centromeric repeat transcription and heterochromatin assembly. Nature. 2008;451:734–737. doi: 10.1038/nature06561. [DOI] [PubMed] [Google Scholar]
- Ellermeier C, Higuchi EC, Phadnis N, Holm L, Geelhood JL, Thon G, Smith GR. RNAi and heterochromatin repress centromeric meiotic recombination. Proc Natl Acad Sci U S A. 2010;107:8701–8705. doi: 10.1073/pnas.0914160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folco HD, Pidoux AL, Urano T, Allshire RC. Heterochromatin and RNAi are required to establish CENP-A chromatin at centromeres. Science. 2008;319:94–97. doi: 10.1126/science.1150944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukagawa T, Earnshaw WC. The centromere: chromatin foundation for the kinetochore machinery. Dev Cell. 2014;30:496–508. doi: 10.1016/j.devcel.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M, He H, Dong Q, Sun S, Li F. Ectopic Centromere Nucleation by CENP-A in Fission Yeast. Genetics. 2014;198:1433–1446. doi: 10.1534/genetics.114.171173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M, He H, Sun S, Li C, Li F. Cell cycle-dependent deposition of CENP-A requires the Dos1/2-Cdc20 complex. Proc Natl Acad Sci U S A. 2013;110:606–611. doi: 10.1073/pnas.1214874110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong EJ, Villen J, Gerace EL, Gygi SP, Moazed D. A cullin E3 ubiquitin ligase complex associates with Rik1 and the Clr4 histone H3-K9 methyltransferase and is required for RNAi-mediated heterochromatin formation. RNA Biol. 2005;2:106–111. doi: 10.4161/rna.2.3.2131. [DOI] [PubMed] [Google Scholar]
- Horn PJ, Bastie JN, Peterson CL. A Rik1-associated, cullin-dependent E3 ubiquitin ligase is essential for heterochromatin formation. Genes & Development. 2005;19:1705–1714. doi: 10.1101/gad.1328005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson DF, Marshall KM, Earnshaw WC. Condensin: Architect of mitotic chromosomes. Chromosome Res. 2009;17:131–144. doi: 10.1007/s10577-008-9009-7. [DOI] [PubMed] [Google Scholar]
- Iida T, Nakayama J, Moazed D. siRNA-mediated heterochromatin establishment requires HP1 and is associated with antisense transcription. Mol Cell. 2008;31:178–189. doi: 10.1016/j.molcel.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S, Kobayashi R, Grewal SI. Ubiquitin ligase component Cul4 associates with Clr4 histone methyltransferase to assemble heterochromatin. Nat Cell Biol. 2005;7:1007–1013. doi: 10.1038/ncb1300. [DOI] [PubMed] [Google Scholar]
- Kim SM, Dubey DD, Huberman JA. Early-replicating heterochromatin. Genes Dev. 2003;17:330–335. doi: 10.1101/gad.1046203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloc A, Zaratiegui M, Nora E, Martienssen R. RNA interference guides histone modification during the S phase of chromosomal replication. Current Biology. 2008;18:490–495. doi: 10.1016/j.cub.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Goto DB, Zaratiegui M, Tang X, Martienssen R, Cande WZ. Two novel proteins, Dos1 and Dos2, interact with Rik1 to regulate heterochromatic RNA interference and histone modification. Current Biology. 2005;15:1448–1457. doi: 10.1016/j.cub.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Li F, Huarte M, Zaratiegui M, Vaughn MW, Shi Y, Martienssen R, Cande WZ. Lid2 Is Required for Coordinating H3K4 and H3K9 Methylation of Heterochromatin and Euchromatin. Cell. 2008;135:272–283. doi: 10.1016/j.cell.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Martienssen R, Cande WZ. Coordination of DNA replication and histone modification by the Rik1-Dos2 complex. Nature. 2011a;475:244–248. doi: 10.1038/nature10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li PC, Chretien L, Cote J, Kelly TJ, Forsburg SL. S. pombe replication protein Cdc18 (Cdc6) interacts with Swi6 (HP1) heterochromatin protein: region specific effects and replication timing in the centromere. Cell Cycle. 2011b;10:323–336. doi: 10.4161/cc.10.2.14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martienssen R, Lippman Z, May B, Ronemus M, Vaughn M. Transposons, tandem repeats, and the silencing of imprinted genes. Cold Spring Harb Symp Quant Biol. 2004;69:371–379. doi: 10.1101/sqb.2004.69.371. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Motamedi MR, Verdel A, Colmenares SU, Gerber SA, Gygi SP, Moazed D. Two RNAi complexes, RITS and RDRC, physically interact and localize to noncoding centromeric RNAs. Cell. 2004;119:789–802. doi: 10.1016/j.cell.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Nakayama J, Rice JC, Strahl BD, Allis CD, Grewal SI. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science. 2001;292:110–113. doi: 10.1126/science.1060118. [DOI] [PubMed] [Google Scholar]
- Oliveira RA, Coelho PA, Sunkel CE. The condensin I subunit Barren/CAP-H is essential for the structural integrity of centromeric heterochromatin during mitosis. Mol Cell Biol. 2005;25:8971–8984. doi: 10.1128/MCB.25.20.8971-8984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard GF, Kerrest A, Dujon B. Comparative genomics and molecular dynamics of DNA repeats in eukaryotes. Microbiol Mol Biol Rev. 2008;72:686–727. doi: 10.1128/MMBR.00011-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka Y, Sutani T, Yamashita Y, Saitoh S, Takeuchi M, Nakaseko Y, Yanagida M. Fission yeast cut3 and cut14, members of a ubiquitous protein family, are required for chromosome condensation and segregation in mitosis. EMBO J. 1994;13:4938–4952. doi: 10.1002/j.1460-2075.1994.tb06821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoshkin A, Arnaoutov A, Jansen LE, Ouspenski I, Dye L, Karpova T, McNally J, Dasso M, Cleveland DW, Strunnikov A. Human condensin function is essential for centromeric chromatin assembly and proper sister kinetochore orientation. PLoS One. 2009;4:e6831. doi: 10.1371/journal.pone.0006831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro JA, von Sternberg R. Why repetitive DNA is essential to genome function. Biol Rev. 2005;80:227–250. doi: 10.1017/s1464793104006657. [DOI] [PubMed] [Google Scholar]
- Simmer F, Buscaino A, Kos-Braun IC, Kagansky A, Boukaba A, Urano T, Kerr AR, Allshire RC. Hairpin RNA induces secondary small interfering RNA synthesis and silencing in trans in fission yeast. EMBO Rep. 2010;11:112–118. doi: 10.1038/embor.2009.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Phatale PA, Sullivan CM, Pomraning KR, Freitag M. Heterochromatin is required for normal distribution of Neurospora crassa CenH3. Mol Cell Biol. 2011;31:2528–2542. doi: 10.1128/MCB.01285-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Chen ES, Yanagida M. Requirement of Mis6 centromere connector for localizing a CENP-A-like protein in fission yeast. Science. 2000;288:2215–2219. doi: 10.1126/science.288.5474.2215. [DOI] [PubMed] [Google Scholar]
- Thon G, Hansen KR, Altes SP, Sidhu D, Singh G, Verhein-Hansen J, Bonaduce MJ, Klart AJS. The Clr7 and Clr8 directionality factors and the Pcu4 cullin mediate heterochromatin formation in the fission yeast Schizosaccharomyces pombe. Genetics. 2005;171:1583–1595. doi: 10.1534/genetics.105.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vader G, Blitzblau HG, Tame MA, Falk JE, Curtin L, Hochwagen A. Protection of repetitive DNA borders from self-induced meiotic instability. Nature. 2011;477:115–119. doi: 10.1038/nature10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe TA, Kidner C, Hall IM, Teng G, Grewal SIS, Martienssen RA. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science. 2002;297:1833–1837. doi: 10.1126/science.1074973. [DOI] [PubMed] [Google Scholar]
- Warburton PE, Hasson D, Guillem F, Lescale C, Jin X, Abrusan G. Analysis of the largest tandemly repeated DNA families in the human genome. BMC Genomics. 2008;9:533. doi: 10.1186/1471-2164-9-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood V, Gwilliam R, Rajandream MA, Lyne M, Lyne R, Stewart A, Sgouros J, Peat N, Hayles J, Baker S, et al. The genome sequence of Schizosaccharomyces pombe. Nature. 2002;415:871–880. doi: 10.1038/nature724. [DOI] [PubMed] [Google Scholar]
- Zaratiegui M, Castel SE, Irvine DV, Kloc A, Ren J, Li F, de Castro E, Marin L, Chang AY, Goto D, et al. RNAi promotes heterochromatic silencing through replication-coupled release of RNA Pol II. Nature. 2011;479:135–138. doi: 10.1038/nature10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.