Abstract
Background
Preparation and processing of free-floating histological sections involve a series of steps. The amount of labor, particularly sectioning and mounting, quickly multiplies as the number of samples increases. Embedding tissue samples in a flexible matrix allows simultaneous handling of multiple samples and preserves the integrity of the tissue during histological processing. However, aligning multiple asymmetrical samples, for example small-animal brains, in a particular orientation requires skillful arrangement and securing of the samples by pinning onto a solid surface. Consequently, costly technical services offered by contract research organizations are often sought.
New Method
An improved approach to align and embed multiple whole or half rodent brain samples into a gelatin-based matrix is described. Using a template specifically designed to form arrayed mouse brain-shaped cavities, a “receiving matrix” is prepared. Inserting brain samples directly into the cavities allows the samples to be effortlessly positioned into a uniform orientation and embedded in a block of matrix.
Results
Multiple mouse brains were arrayed in a uniform orientation in a gelatin matrix block with ease using the receiving matrix. The gelatin-embedded brains were simultaneously sectioned and stained, and effortlessly mounted onto glass slides.
Comparison with Existing Methods
The improved approach allowed multiple whole or half mouse brains to be easily arrayed without pinning the samples onto a solid surface and prevented damages or shifting of the samples during embedding.
Conclusions
The new approach to array multiple brain samples provides a simple way to prepare gelatin-embedded whole or half brain arrays of commercial quality.
Keywords: Brain array, tissue preparation, gelatin, embedding, matrix, frozen-section, immunohistochemistry
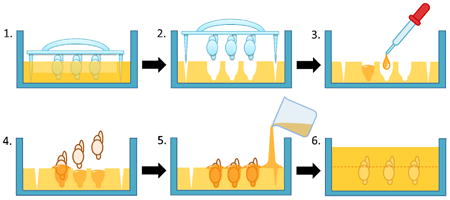
1. Introduction
Histological analysis of biological tissue is essential for studying cellular morphology and phenotypes in situ. However, the process to prepare microscope-ready tissue samples is rather laborious, and more time and effort are required when a large number of tissue samples needs to be analyzed. In clinical pathology laboratories, tissue microarray, a method to “punch” out pieces of tissues and embed them in paraffin in an ordered arrangement, has been developed and is often utilized for simultaneous analysis of multiple biopsied specimens (Kononen, et al., 1998; Miettinen, 2012). In basic biomedical research, where the analysis of whole organ tissues from experimental animals may be preferred, tissue embedding techniques have been more commonly used to protect the structure of complex organs such as the brain (Bjarkam, et al., 2001; Griffioen, et al., 1992;) and cochlea (Hurley, et al., 2003) during processing rather than to process multiple samples at the same time.
Recently, Smiley and Bleiwas (2012) have described a method to embed multiple mouse brains. In their protocol, the brain samples were secured on a solid surface with pins and embedded in a gelatin-based matrix containing several ingredients including albumin, lysine and glutaraldehyde. While they successfully demonstrated simultaneous sectioning and staining of multiple brain samples, the method requires each brain sample to have an extra tissue area (e.g., brainstem) to be pinned or risk damaging a portion of the sample by the pinning process. This requirement poses a challenge when smaller samples such as neonatal or half-brain samples must be secured. Furthermore the inclusion of additional constituents to facilitate cross-linking of gelatin to the tissue samples makes the preparation of the matrix more complicated and costly.
In this report, we describe a new method to effortlessly align and embed multiple brain samples in a gelatin-based matrix block for simultaneous tissue processing. With two prototypes of specifically designed “brain array” casts, we first created a mold or “sample receiving matrix” with sample-shaped cavities in an organized arrangement. Using this technique, we were able to eliminate the pinning process and align small, mouse brain hemispheres without damaging any part of the tissue. The gelatin block with embedded samples can be ready for frozen sectioning within 3-4 days without many of the additional ingredients included in previously described embedding matrices (Smiley and Bleiwas, 2012).
2. Materials and Methods
2.1. Materials
Gelatin (from porcine skin, type A, gel strength 300) was obtained from Sigma-Aldrich Co. (St. Louis, MO, USA) and used to make a gelatin-based embedding matrix. “Brain array casts” were specifically designed in order to align multiple mouse brains and embed them in a single block. Two prototypes tested in this study were constructed with high heat-sensitive polymer (high-temperature melting glue gun glue) and liquid rubber (Fig. 1). The rabbit polyclonal anti-mouse Iba-1 antibody was purchased from Wako Chemicals USA (Richmond, VA, USA) and used at a 1:1000 dilution. For immunohistochemistry, the anti-rabbit VECTASTAIN Elite ABC kit and ImmPact VIP were obtained from Vector Laboratories (Burlingame, CA, USA). The Tissue-Tek Cryo-O.C.T. compound was purchased from Fisher Scientific (Pittsburgh, PA, USA). All other chemicals were also purchased from Sigma-Aldrich (St. Louis, MO, USA).
Figure 1. Brain array cast prototypes.
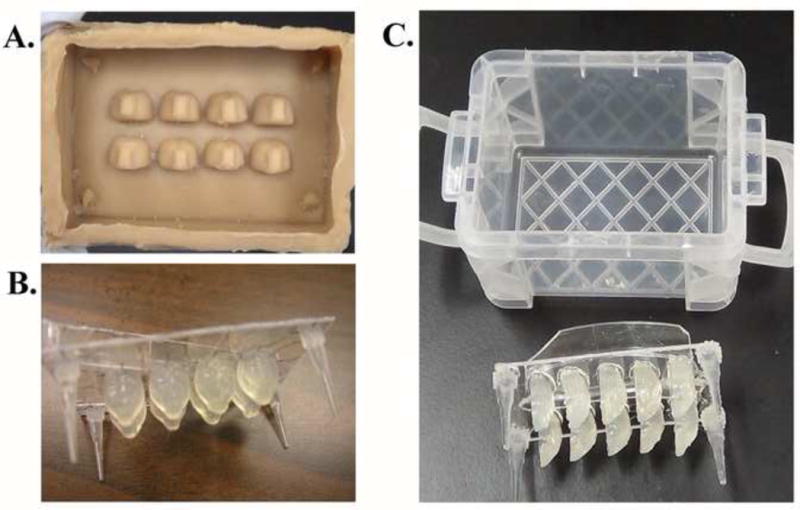
Devices for preparing sample receiving matrices were designed and prototypes were created from liquid rubber (A) and heat-sensitive polymer (B & C). The designs A and B create cavities that are the shape of whole brains, while design C forms half-brain shaped cavities in a sample receiving matrix. Design C, pictured with a small plastic container used as a receptacle, was used to demonstrate the embedding procedure.
2.2. Animal tissues
Whole brains were collected from 2 or 10 month-old C57BL/6 mice housed with a 12 h light-12 h dark schedule. These animals were part of another independent study performed under the approval of the Institutional Animal Care and Use Committee of the University of North Dakota. The animals were sacrificed at the completion of the study by CO2 asphyxiation followed by transcardiac perfusion of phosphate-buffered saline (PBS) and 4% paraformaldehyde (PFA). The brains were carefully removed and post-fixed in 4% PFA at 4°C for 2 days, followed by sequential equilibration in 15% and 30% sucrose-containing PBS.
2.3. Gelatin matrix
Gelatin powder was dissolved in PBS to make 15% (w/v) solution by leaving the mixture in a capped bottle for several hours in a laboratory dry oven at 40-45 °C, or in a water bath at 40-42 °C. The bottle was intermittently mixed by gentle inversion to facilitate dissolving of the gelatin powder. After all the gelatin powder was in solution, 0.02% sodium azide was added to the gelatin matrix as a preservative. A “sample receiving matrix” was made by slowly pouring the gelatin matrix solution into a liquid rubber cast (Fig. 2A-1), or alternatively into a small container and subsequently inserting a “brain array cast” into the matrix to avoid creating air bubbles (Fig. 2B-1). The gelatin matrix was allowed to solidify at room temperature, and placed at 4 °C for 15 min or more to further harden the matrix for easier removal of the mold. The brain array cast was gently removed from the matrix to complete a sample receiving matrix with brain-shaped cavities (Fig.2A-2 & B-2).
Figure 2. Preparation of sample receiving matrices using brain array cast prototypes.
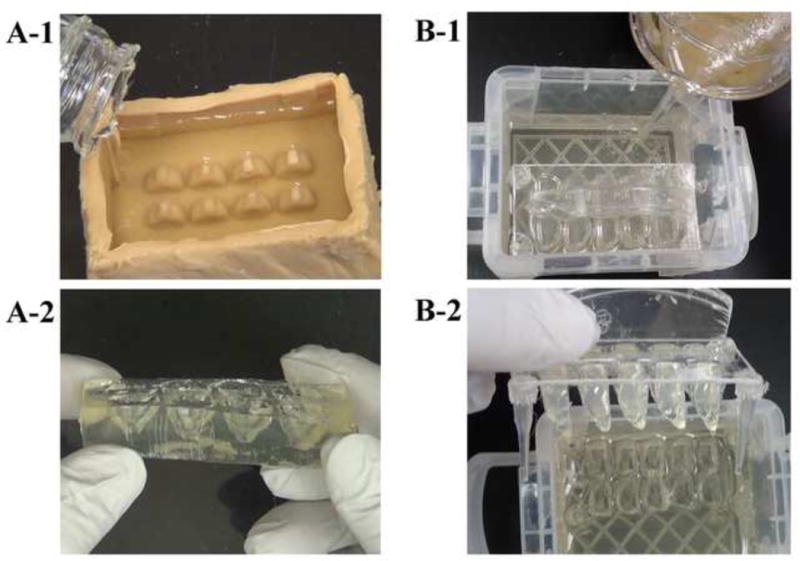
Two prototype brain array casts were used to prepare sample receiving matrices for whole brains (A) and half brains (B) by pouring 15% gelatin in PBS (A-1 & B-1). A-2 and B-2 show the whole or half brain-shaped cavities in respective sample receiving matrices after removal of the casts.
2.4. Tissue embedding
PFA-fixed, sucrose-equilibrated brain samples were incubated in the gelatin matrix solution for 1 hr at 37-39 °C prior to placing the samples in the receiving matrix (Fig. 3A). A completed receiving matrix was placed in container deep enough to hold the receiving matrix and the length of the brain samples. After allowing the sample receiving matrix to reach room temperature, a matrix-infused brain sample was inserted into each brain-shaped cavity immediately after the cavity was partially filled with molten gelatin matrix (Fig. 3B). This process was repeated until all brain samples to be grouped were placed in their respective cavity. Once the brain samples were secured in their places, gelatin matrix was poured to cover the brain samples, avoiding introduction of air bubbles. The gelatin block was allowed to set completely by cooling at room temperature and then at 4 °C until firm before carefully being removed from the container (Fig. 3C & D).
Figure 3. Sample embedding procedure.
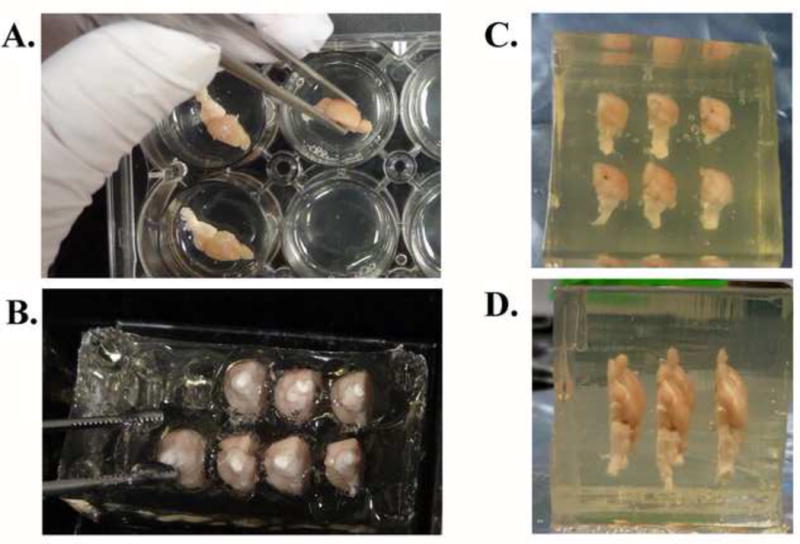
Half-brain specimens were incubated in the molten gelatin matrix solution at 37-39 °C for 1 hr (A) before being inserted into a sample receiving matrix for half-brain samples (B). Once all of the samples to be included in one gelatin block were placed at the desired positions, the gelatin matrix solution was poured to cover the samples and allowed to harden. A completed and trimmed block encasing six half-brain specimens is shown in rostral (C) and ventral (D) views.
2.5. Preparation of gelatin block for frozen sectioning
The resulting gelatin block containing multiple brain samples was trimmed with a razor blade and placed in 4% PFA at 4 °C for 24h, then sequentially equilibrated in 15% sucrose in PBS (w/v) for 1 day and in 30% sucrose in PBS (w/v) for 2 days. The block was kept in the 30% sucrose in PBS until used. For frozen sectioning on a sliding microtome (Leica SM2000 R, Leica Biosystems, Nussloch, Germany) the fixed and sucrose-equilibrated gelatin block was dabbed dry with laboratory tissue wipes and quickly frozen by immersing in 2-methylbutane/dry ice mixture for approximately 20 seconds. The frozen block was removed from 2-methylbutane and kept in dry ice until mounted on the microtome stage using Cryo-O.C.T. compound. The gelatin block was sectioned to produce 40 μm-thick, free-floating sections (Fig. 4). Gelatin block sections were collected in PBS for immediate processing, or stored long-term at -20 °C in cryoprotectant solution containing 30% sucrose (w/v) and 30% ethylene glycol (v/v) in 0.1 M sodium phosphate buffer (pH 7.4).
Figure 4. Frozen sectioning of a gelatin block containing seven half-brain specimens.
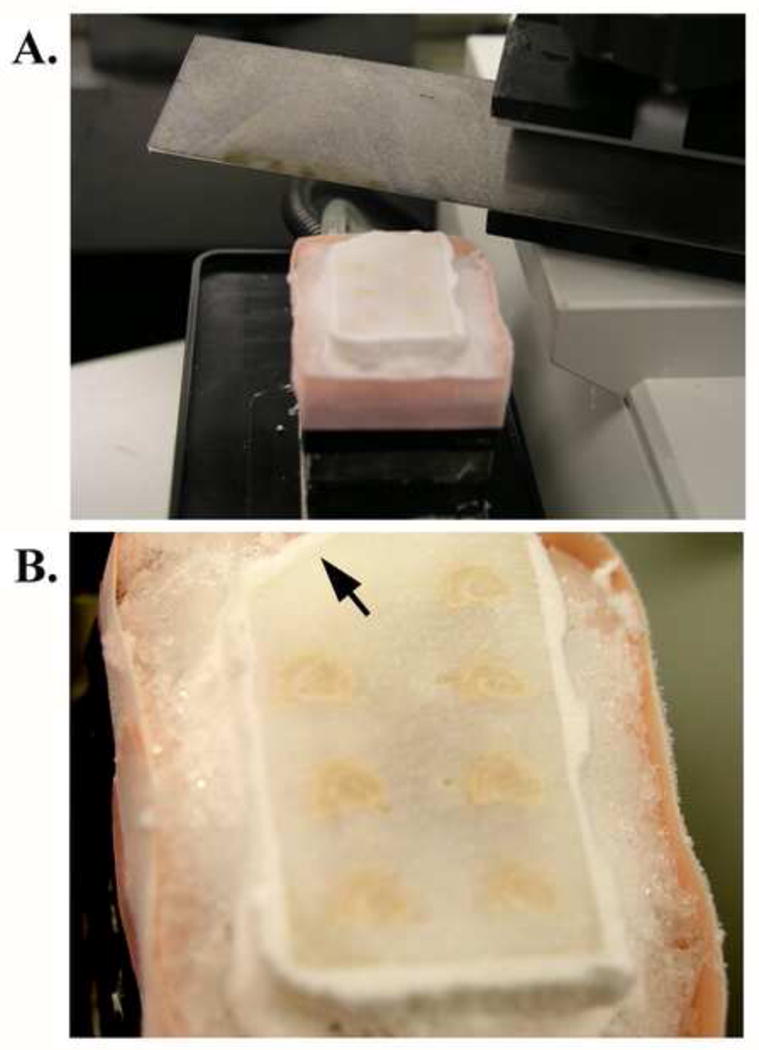
The gelatin block was rapidly frozen in a 2-methylbutane/dry ice mixture for approximately 20 seconds and kept in dry ice until mounted on to the microtome stage with O.C.T. compound. The temperature of the block was maintained by the freezing stage temperature controller, and with additional dry ice when needed, during sectioning. A frozen block on the microtome stage is shown (A). A corner of the block was notched for the purpose of identification when working with more than one block (arrow in B).
2.6. Immunostaining gelatin block sections
Prior to processing for immunostaining procedures, the gelatin block sections that had been stored in the cryoprotectant were rinsed thoroughly in PBS (15 min × 4). Immunostaining was performed in the same manner as with any free-floating tissue sample (Nagamoto-Combs, et al., 2010). Upon completion of immunostaining, sections were floated in double-distilled H2O and mounted onto gelatin-subbed glass slides. Any wrinkles and folds in the sections were smoothed with a paint brush, and the slides were air-dried completely before undergoing the dehydration and coverslipping procedures (Nagamoto-Combs, et al., 2010).
2.7. Microscopy and Photography
Gelatin embedded tissues were observed under an Olympus BX60 microscope (Olympus America, Inc., Center Valley, PA, USA) with 4×, 10×, 20×, and 40× objectives. Photomicrographs were taken using a SPOT RT Slider digital camera and software (Diagnostic Instruments, Inc., Sterling Heights, MI, USA) and formatted with Adobe Photoshop software (Adobe Systems Inc., San Jose, CA, USA). Other photographs were taken using Olympus digital camera C-5050 (Olympus America, Inc.).
3. Results
3.1. Brain array cast prototypes efficiently created “sample receiving matrix” with multiple close-fitting brain-shaped cavities that securely hold brain tissue
To develop a technique to simplify aligning and embedding of multiple brain tissue samples, two designs of brain array cast prototypes were tested (Fig. 1, A & B). Both prototypes used produced functional sample receiving matrix, providing well-aligned, brain-shaped impressions (Fig. 2A-2 & B-2). By comparison the removal of the sample receiving matrix was easier with the liquid-rubber based brain array cast. The polymer-based brain array cast had a higher tendency to adhere to the gelatin matrix once it was set, although it was possible to remove the sample receiving matrix after carefully separating the matrix from the cast with a flat spatula (Fig 2B-2). In both cases, cooling the gelatin matrix at 4 °C facilitated the removal of fully intact sample receiving matrix.
3.2. Incubation of brain samples in the gelatin matrix prior to embedding provided better adhesion of the tissue to the matrix
Detachment of tissue samples from the gelatin matrix during histological processing had previously been a major issue in our hands, which resulted in sample disarray. Based on a gelatin infusion technique to preserve the morphology of cochlear tissue (Hurley, et al., 2003), we incubated PFA-fixed, sucrose-equilibrated brain samples in the gelatin matrix for 1 hr at 37-39 °C prior to embedding. This step allowed the gelatin matrix to permeate into cisterns and fissures of the brain samples and ensured adhesion of the matrix to the sample even after sectioning (Fig. 5).
Figure 5. Immunostained and slide-mounted gelatin-embedded brain sections.
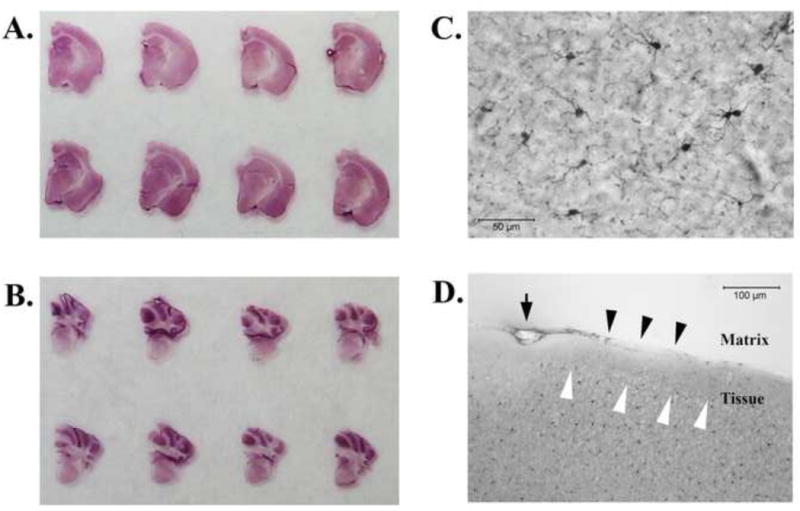
The brain sections were effortlessly mounted onto glass slides as one intact sheet as arranged in the gelatin matrix (A & B). A higher magnification (40×) showed distinct morphology of Iba1- immunoreactive microglia in the mouse brains (C, a region in the cerebral cortex). Some parts of brain sections exhibited uneven staining patterns around the edges of tissues (D) that appeared lighter (black arrowheads) or darker (white arrowheads). The downward arrow in D indicates an air bubble unintentionally created between the tissue and the matrix. Scale bars: 50 μm (C); 100 μm (D).
3.3. Sample receiving matrix allowed effortless alignment of multiple brain tissues in a single gelatin matrix block
The brain-shaped cavities created in the sample receiving matrix simplified the arrangement of brain samples by providing well-fitting, distinct “place holders” for the samples. Although brain samples were placed vertically into the sample receiving matrix, the samples did not tip over indicating that the cavities provided enough depth for the brain samples to stay upright. However, the overall size of the cavities, which were created by the fresh-brain size models, were slightly larger than the sucrose-equilibrated brain samples. Pre-filling the cavities with gelatin matrix solution before insertion of brain samples helped better control tipping of the samples and limited trapping of air bubbles. Pouring molten gelatin matrix over the samples placed in the impression of the receiving matrix did not cause floatation or derangement of the sample tissues (Fig. 3C & D).
3.4. Fixed and sucrose-equilibrated gelatin blocks can be frozen sectioned to prepare free-floating tissue samples
Sectioning frozen gelatin blocks at 40 μm on a sliding microtome did not cause any difficulty when the temperature of the block was kept around -28 to -25 °C. While gelatin sections thawed and folded as the microtome blade cut through the gelatin block, they rapidly unfolded as soon as they were placed in PBS or cryoprotectant solution. The thawed sections were durable and did not tear or release sample tissues during handling with a paint brush or immunostaining procedures.
3.5. Gelatin matrix did not interfere with immunohistochemical identification of microglia with anti-Iba1 antibody
Staining gelatin matrix sections required fewer staining compartments since having multiple tissue samples fixed in an arrangement circumvented the need to keep different samples separated during antibody incubation and rinsing. The gelatin matrix did not appear to adsorb antibodies or show background staining (Fig. 5A & B) as we sometimes observed using albumin-containing embedding matrix (data not shown). Importantly, embedding the brain samples did not appear to interfere with immunohistochemical staining of Iba1, which showed distinct microglia morphology (Fig. 5C). Although we sometimes observed uneven staining patterns around limited edges of brain sections (Fig. 5D, black and white arrowheads), most regions of the brains were unaffected. Sodium azide (0.02% w/v) added into the gelatin matrix solution as a preservative also did not have any apparent effect, at least for the antigen tested.
3.6. Gelatin-embedding allowed effortless mounting of multiple tissue sections on glass slides and did not show tissue shrinkage
Gelatin matrix sections were particularly easy to mount onto histological slides, circumventing the need to handle each tissue section. The matrix and tissues air-dried evenly and did not generate shrinkage-associated cracks (Fig. 5).
4. Discussions and Conclusions
Two prototypes of brain array casts that differed in the materials (liquid rubber vs. heat-sensitive polymer) and designs (built-in container vs. casting insert plus container) were tested (Fig. 1A & B). Both designs successfully produced effective sample receiving matrices with brain-shaped cavities that securely held multiple brain samples in a vertical position during embedding. Removal of sample receiving matrix from the cast constructed from liquid rubber required less effort than that made from heat-sensitive glue-stick polymer, since the latter material adhered more closely to the gelatin matrix and required careful prying with a fine spatula. Non-stick substances, such as silicone rubber, will therefore be better suited materials to construct the brain array cast and it is currently under development. Furthermore, the containerlike design of the liquid rubber cast was more convenient since it did not require an additional receptacle.
In addition to the material and shape of the brain array cast, the brain-shaped impression should also be improved. Since the brain models on the brain array casts were constructed based on the size of a freshly isolated mouse brain, the brain-shaped cavities tend to be slightly larger than the sucrose-equilibrated brain samples. Reducing the size of the cavities will provide better fitting of the samples, thus preventing unwanted shifting of samples during embedding. However, due to the elasticity of solidified gelatin and its semi-liquid state at a warmer temperature, the brain-shaped cavity of the matrix is relatively forgiving to the variable shapes and sizes of brain samples. For example, if a sample is smaller than the cavity, the extra space in the cavity can be simply filled with additional, partly-solidified gelatin matrix solution. Conversely when the sample is slightly larger than the cavity, insertion of a sample pre-incubated at 37-39 °C (Materials and Methods, section 2.4) gently stretches the cavity. Nonetheless, brain array casts with different size cavities that accommodate the samples from various age groups would ultimately be ideal.
From these observations, we conclude that a brain array cast made from silicone rubber or other non-stick materials with custom-sized brain models in the container-like design will provide the most effective device to prepare a sample receiving matrix. We are currently working to optimize the brain array cast.
Gelatin is an excellent medium to embed tissue samples. At around 37-39 °C, 15% gelatin is liquid; therefore infusion can be carried out without scalding the samples. Once solidified, the gelatin block became very sturdy but remained flexible, allowing further trimming of the block, if necessary, to ensure the final sections fit glass slides. Trimmed gelatin pieces were re-melted and used again to minimize waste. Frozen-sectioned gelatin matrix demonstrated a texture similar to fixed tissue, and no special handling strategy was necessary. Incubation of the tissue samples in the gelatin matrix prior to embedding greatly improved adherence of the tissue sections to the matrix after frozen sectioning and throughout histological procedures. Although some edge portions of brain sections exhibited slightly uneven staining, the overall imunostaining were not significantly affected by the embedding. The “edge effect” may be due to the infusion of gelatin or sodium azide in the matrix into the tissue, and it may be reduced by adjusting the embedding procedure with shorter infusion time, lower gelatin concentrations and/or removal of the preservative from the matrix.
In conclusion, our brain array cast prototypes can be used as a convenient tool to effortlessly align and embed multiple brain samples in a gelatin block. Furthermore, the use of gelatin in PBS serves as a cost and time effective embedding matrix composition. Embedding multiple brain samples into one gelatin block expedites tissue processing and facilitates histological analyses.
Highlights.
An improved method to align and embed multiple rodent brains in a gelatin-based matrix was developed to facilitate histological processing.
Templates were designed to form brain-shaped cavities in a gelatin block in order to create a “receiving matrix” to hold multiple brain samples in an organized manner.
The receiving matrix simplified and accelerated alignment and embedding of multiple brain samples in a block of gelatin matrix.
Multiple brain samples were sectioned, stained and mounted simultaneously, thus shortening the tissue processing time typically takes to handle multiple samples individually.
Acknowledgments
This work was supported by the Department of Pathology, University of North Dakota School of Medicine and Health Sciences and NIH grant number 5R01AG042819.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bjarkam CR, Pedersen M, Sorensen JC. New strategies for embedding, orientation and sectioning of small brain specimens enable direct correlation to MR-images, brain atlases, or use of unbiased stereology. J Neurosci Methods. 2001;108(2):153–9. doi: 10.1016/s0165-0270(01)00383-1. [DOI] [PubMed] [Google Scholar]
- Griffioen HA, Van der Beek E, Boer GJ. Gelatin embedding to preserve lesion-damaged hypothalami and intracerebroventricular grafts for vibratome slicing and immunocytochemistry. J Neurosci Methods. 1992;43(1):43–7. doi: 10.1016/0165-0270(92)90065-l. [DOI] [PubMed] [Google Scholar]
- Hurley PA, Clarke M, Crook JM, Wise AK, Shepherd RK. Cochlear immunochemistry--a new technique based on gelatin embedding. J Neurosci Methods. 2003;129(1):81–6. doi: 10.1016/s0165-0270(03)00211-5. [DOI] [PubMed] [Google Scholar]
- Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4(7):844–7. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- Miettinen M. “A simple method for generating multitissue blocks without special equipment”. Appl Immunohistochem Mol Morphol. 2012;20(4):410–2. doi: 10.1097/PAI.0b013e318245c82f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamoto-Combs K, Morecraft RJ, Darling WG, Combs CK. Long-term gliosis and molecular changes in the cervical spinal cord of the rhesus monkey after traumatic brain injury. J Neurotrauma. 2010;27(3):565–85. doi: 10.1089/neu.2009.0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Bleiwas C. Embedding matrix for simultaneous processing of multiple histological samples. J Neurosci Methods. 2012;209(1):195–8. doi: 10.1016/j.jneumeth.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


