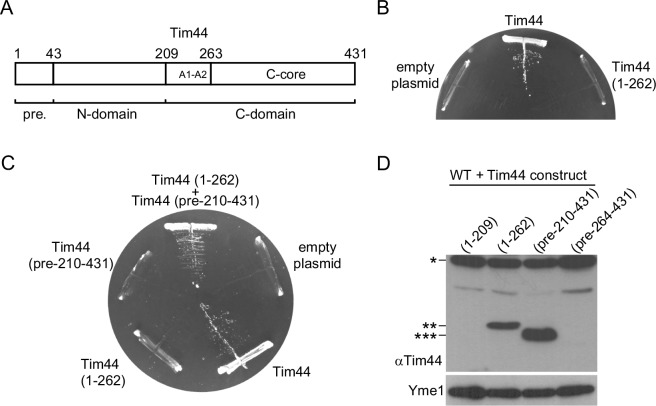
Figure 1. The function of Tim44 can be rescued by its two domains expressed in trans but not by either of the domains alone.
(A) Schematic representation of Tim44 domain structure (numbering according to yeast Tim44 sequence). pre. - presequence (B and C) A haploid yeast deletion strain of TIM44 carrying the wild-type copy of TIM44 on a URA plasmid was transformed with centromeric plasmids carrying indicated constructs of Tim44 under control of endogenous promoter and 3'UTR. Cells were plated on medium containing 5-fluoroorotic acid and incubated at 30°C. The plasmid carrying wild-type Tim44 and an empty plasmid were used as positive and negative controls, respectively. (D) Total cell extracts of wild-type yeast cells transformed with plasmids coding for indicated Tim44 constructs under GPD promoter were analysed by SDS–PAGE and immunoblotting against depicted antibodies. *, ** and *** - protein bands detected with antibodies raised against full-length Tim44.