Table 1.
Antimicrobial activity of synthesized platensimycin analogue.
| Sl. number | Analogues name | Analogues structure | Antimicrobial activity | Reference |
|---|---|---|---|---|
| 1 | (−)-Platencin |
|
Inhibits both FabF and FabH with similar potency | [12] |
|
| ||||
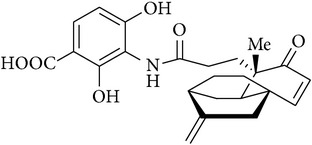
| 2 | 7-Phenylplatensimycin |
|
MIC against S. aureus (MSSA), 0.25 μg/mL | [15] |
|
| ||||
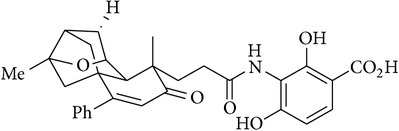
| 3 | 11-Methyl-7-phenylplatensimycin |
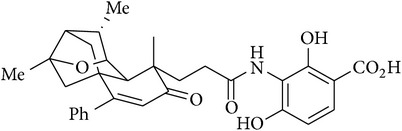
|
MIC against S. aureus (MSSA), <0.25 μg/mL | [15] |
|
| ||||
| 4 | Sulfonamide analogues of platensimycin |
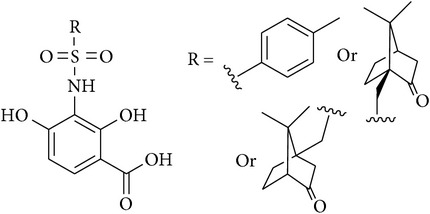
|
Not tested | [16] |
|
| ||||
| 5 | Oxazinidinyl platensimycin |
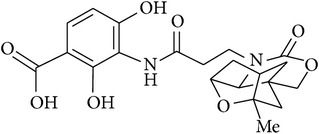
|
MIC of 90 μg/mL against S. aureus, S. agalactiae, and B. subtilis | [17] |
|
| ||||
| 6 | Isoplatencin |
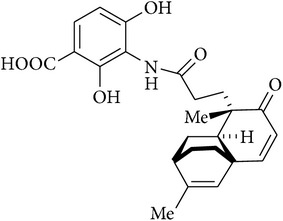
|
MIC against S. aureus (MSSA), 0.4 μg/mL | [18] |
|
| ||||
| 7 | Cl-iso-platencin |
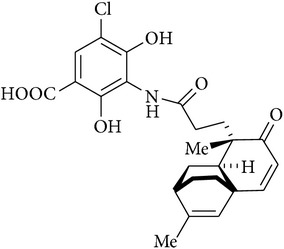
|
MIC against S. aureus (MSSA), >25.6 μg/mL | [18] |
|
| ||||
| 8 | Cl-platencin |
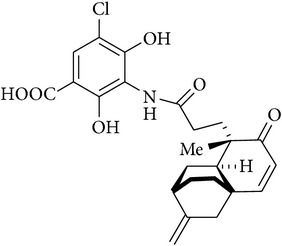
|
MIC against S. aureus (MSSA), >25.6 μg/mL | [18] |
|
| ||||
| 9 | Dehydrohomoplatencin |
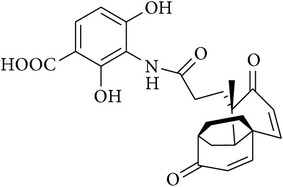
|
MIC against S. aureus (MSSA), 0.4 μg/mL | [19] |
|
| ||||
| 10 | Isoplatensimycin |
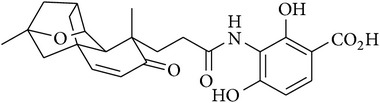
|
MIC against S. aureus (MSSA), 128 μg/mL | [20] |
|
| ||||
| 11 | Carbaplatensimycin |
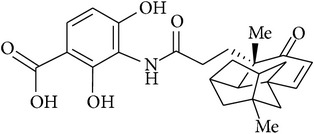
|
MIC against S. aureus, 0.4–1.1 μg/mL | [21] |
|
| ||||
| 12 | Platensimycin B1 and B3 |
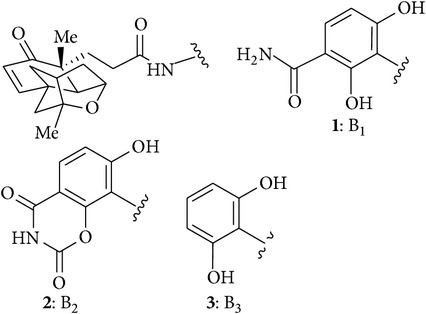
|
Not tested | [22] |
|
| ||||
| 13 | Platensimide A |
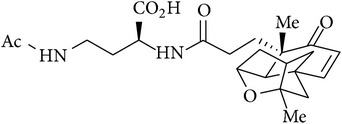
|
Not tested | [22] |
|
| ||||
| 14 | Homoplatensimide A and homoplatensimide A methyl ester |
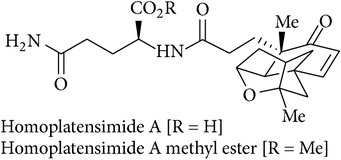
|
Not tested | [22] |
|
| ||||
| 15 | Dialkylamino-2,4-dihydroxybenzoic acids analogues of Platensimycin |
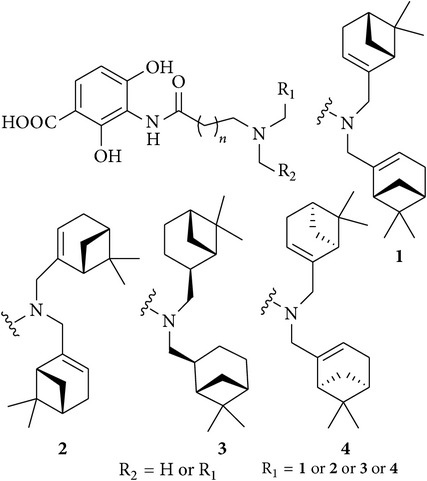
|
Out of 18 Synthesized derivatives, four derivatives which have the substitution 1, 2, 3, and 4, respectively, have shown potential activity against B. subtilis (MIC 2–8 μg/mL) | [23] |
|
| ||||
| 16 | (−)-nor-platencin |
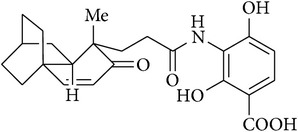
|
MIC against S. aureus (MSSA), 5 μg/mL | [24] |
|
| ||||
| 17 | Adamantaplatensimycin |
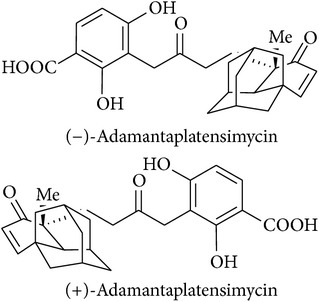
|
MIC of (−)-adamantaplatensimycin against S. aureus (MSSA), 1.3–1.8 μg/mL; MIC of (+)-adamantaplatensimycin against S. aureus (MSSA), >88 μg/mL | [25] |
