Abstract
In previous studies, we have shown that acetylcholinesterase inhibitors and phosphodiesterase inhibitors (PDE-Is) are able to improve object memory by enhancing acquisition processes. On the other hand, only PDE-Is improve consolidation processes. Here we show that the cholinesterase inhibitor donepezil also improves memory performance when administered within 2 min after the acquisition trial. Likewise, both PDE5-I and PDE4-I reversed the scopolamine deficit model when administered within 2 min after the learning trial. PDE5-I was effective up to 45 min after the acquisition trial and PDE4-I was effective when administered between 3 and 5.5 h after the acquisition trial. Taken together, our study suggests that acetylcholine, cGMP, and cAMP are all involved in acquisition processes and that cGMP and cAMP are also involved in early and late consolidation processes, respectively. Most important, these pharmacological studies suggest that acquisition processes continue for some time after the learning trial where they share a short common time frame with early consolidation processes. Additional brain concentration measurements of the drugs suggest that these acquisition processes can continue up to 4–6 min after learning.
Memory is a highly complex cognitive function. The formation of memories requires a sequence of complementary processes to register, maintain, and retrieve events. Therefore, it is common to segregate memory processes into an acquisition, consolidation, and retrieval phase. When testing cognition enhancing properties of drugs, it is particularly relevant to dissociate its effects on acquisition, consolidation, and retrieval, particularly given the accumulating evidence that these different memory processes rely on distinct underlying neurobiological mechanisms (Izquierdo et al. 2006; Winters et al. 2008). This would not only lead to a better understanding of the properties of a drug, but also of neurobiological mechanisms underlying memory processes.
Memory can be tested in wide array of behavioral animal paradigms. However, few of these models allow for an evaluation of effects on the different memory subprocesses. One-trial learning paradigms, such as the passive avoidance test, fear conditioning, and the object recognition test (ORT) enable researchers to evaluate memory-enhancing effects of drugs on individual memory components. Typically, these tests consist of an acquisition trial in which information is presented, and a test trial in which the information has to be retrieved. In these paradigms, the timing of drug administration determines which memory process is affected (Abel and Lattal 2001; van Goethem et al. 2012). That is, when a drug is administered before the acquisition trial, it is capable of influencing acquisition processes, whereas if the drug is given after the acquisition trial it can be assumed to affect consolidation, because acquisition processes are already completed. On the same rationale, drug administration shortly before the test trial will only have an effect on retrieval.
In a previous study, using the object recognition task, we showed that cholinesterase inhibitors (AChE-Is) metrifonate and donepezil enhanced acquisition processes but not consolidation processes, likely to be mediated via an increase in the neurotransmitter acetylcholine (ACh) in the synaptic cleft (Prickaerts et al. 2005). On the other hand, phosphodiesterase type 5 inhibitors (PDE5-Is) affect acquisition and early consolidation processes via an increase in intracellular cyclic guanosine monophosphate (cGMP)/protein kinase G (PKG) signaling, when administered before or directly after learning, respectively (Devan et al. 2004, 2007; Prickaerts et al. 2005; Domek-Lopacinska and Strosznajder 2008; van Donkelaar et al. 2008; Bollen et al. 2014). Also, we found that the phosphodiesterase type 4 inhibitor (PDE4-I) rolipram, which increases cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling, improves both acquisition and late consolidation processes when administered before or at 3 h after learning (Rutten et al. 2006, 2007; Bollen et al. 2014). These findings provide further support for the notion that acquisition and consolidation processes can be associated with dissociable neurobiological processes.
To more accurately determine the exact time windows of acquisition and consolidation processes occur, we administered donepezil, vardenafil, or rolipram at several specific time points after the acquisition trial. The capability of each drug to improve acquisition and/or consolidation was tested by assessing its potential to prevent natural forgetting in a 24-h intertrial interval. Additionally, we tested the effects of the drugs using a 1-h interval against the muscarinic receptor antagonist scopolamine, which is commonly used as a memory deficit model and specifically blocks acquisition processes (Rutten et al. 2006; Winters et al. 2006; Klinkenberg and Blokland 2010). Furthermore, plasma and brain concentrations of the cognition enhancing drugs were measured at several time points after injection, to find out their relationship with the onset of their behavioral effects.
Results
Effects of donepezil
The effects of donepezil treatment on the exploration measures are shown in Table 1. In the 1-h delay interval combined with scopolamine, donepezil and/or scopolamine did not affect the object exploration times (F(6,49) = 1.84, n.s.) in T1 and T2 (F(6,49) = 1.20, n.s.). Similarly, after the 24-h retention delays, no effects of donepezil were found on the exploration levels in T1 (F(8,63) = 1.27, n.s.) and T2 (F(8,63) = 1.11, n.s.).
Table 1.
Treatment conditions, exploration levels (mean (±SEM)), and number (n) of rats in the object recognition task
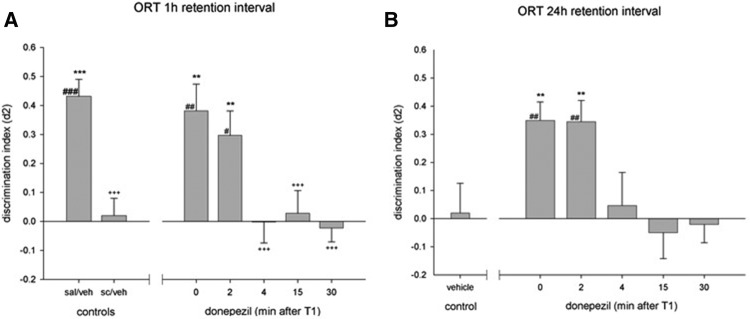
Significant between-group differences on d2 were found in the scopolamine deficit model (F(8,63) = 7.67, P < 0.001) and natural forgetting paradigm (F(8,63) = 3.49, P < 0.01), using one-way ANOVAs. When injected after scopolamine treatment, donepezil attenuated the scopolamine-induced deficit when injected directly up to 2 min after T1, but not when injected after this time point (see Fig. 1A). This shows that donepezil treatment improved memory performance after a 24-h retention delay when injected up to 2 min after T1, but not when injected at 4 min or longer post-T1 intervals (Fig. 1B).
Figure 1.
Effects of donepezil treatment on discrimination performance in an object recognition task using a 1-h interval in combination with scopolamine (A) or a 24-h retention interval (B). Asterisks indicate a difference from scopolamine/vehicle-treated (A) or vehicle-treated (B) animals, least significant difference (LSD): (**) P < 0.01; (***) P < 0.001, plus signs indicate a difference from the saline/vehicle-treated animals, +++P < 0.001 and differences from zero are indicated with hashes, one-sample t-test, #P < 0.05; ##P < 0.01; ###P < 0.001.
Effects of vardenafil
Table 1 shows the effects of vardenafil treatment on the exploration measures. In the 24-h retention delays, vardenafil did not affect object exploration in T1, (F(8,63) = 1.83, n.s.), but exploration differences were found in T2, (F(8,63) = 2.13, P < 0.05). However, post-hoc analysis (LSD) showed no significant differences in e2 between the vardenafil conditions and vehicle.
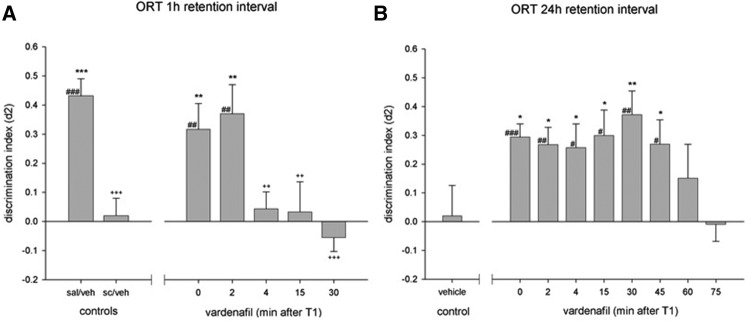
Vardenafil reversed the scopolamine-induced deficits only when injected immediately or 2 min after T1 (see Fig. 2A). Differences in d2 measures were found between groups in the 24-h delay interval, (F(8,63) = 2.48, P < 0.05). Vardenafil treatment improved memory performance in the 24-h delay interval, when applied between 0 until 45 min after T1, but not when injected at a later time point (Fig. 2B).
Figure 2.
Effects of vardenafil treatment on discrimination performance in an object recognition task using a 1 h interval in combination with scopolamine (A): adapted from a previous study (Akkerman et al. 2015) or a 24-h retention interval (B). Asterisks indicate a difference from scopolamine/vehicle-treated (A) or vehicle-treated (B) animals, LSD, (*) P < 0.05; (**) P < 0.01; (***) P < 0.001, plus signs indicate a difference from the saline/vehicle-treated animals (A), LSD, ++ P < 0.01; +++ P < 0.001 and differences from zero are indicated with hashes, one-sample t-test, # P < 0.05; ## P < 0.01; ### P < 0.001.
Effects of rolipram
The effects of rolipram treatment on the exploration indices are shown in Table 1. In the 1-h retention sessions, there were no differences in exploration during T1, (F(6,49) = 1.51, n.s.); however, exploration differences were present in T2, (F(6,49) = 3.06, P < 0.05). Post-hoc analysis (LSD) revealed that all rolipram-treated conditions had lower exploration levels in T2, compared to the vehicle condition. In the 24-h retention intervals, rolipram did not affect object exploration in T1 and T2, (F(5,42) = 2.09, n.s.) and (F(5,42) = 0.16, n.s.), respectively.
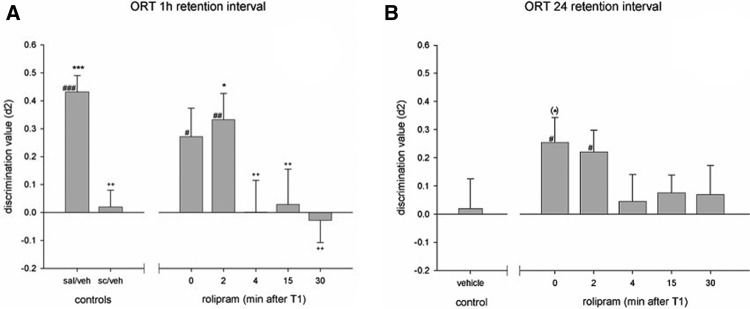
Significant differences were found in discrimination performance between the treatment conditions of the 1-h retention experiment, (F(6,49) = 4.06, P < 0.01). Post-hoc analysis (LSD) revealed that only animals that received rolipram treatment at 2 min after T1 had significantly higher discrimination compared to vehicle-treated animals (Fig. 3A). No significant differences were found (F(5,42) = 1.18, n.s.) between treatment conditions in the 24-h retention experiment (Fig. 3B).
Figure 3.
Effects of rolipram treatment on discrimination performance in an object recognition task using a 1-h interval in combination with scopolamine (A) or a 24-h retention interval (B). Asterisks indicate a difference from scopolamine/vehicle-treated (A) or vehicle-treated (B) animals, LSD, (*) P = 0.062; (*) P < 0.05; (***) P < 0.001, plus signs indicate a difference from the saline/vehicle-treated animals (A), LSD, ++ P < 0.01 and differences from zero are indicated with hashes, one-sample t-test, # P < 0.05; ## P < 0.01; ### P < 0.001.
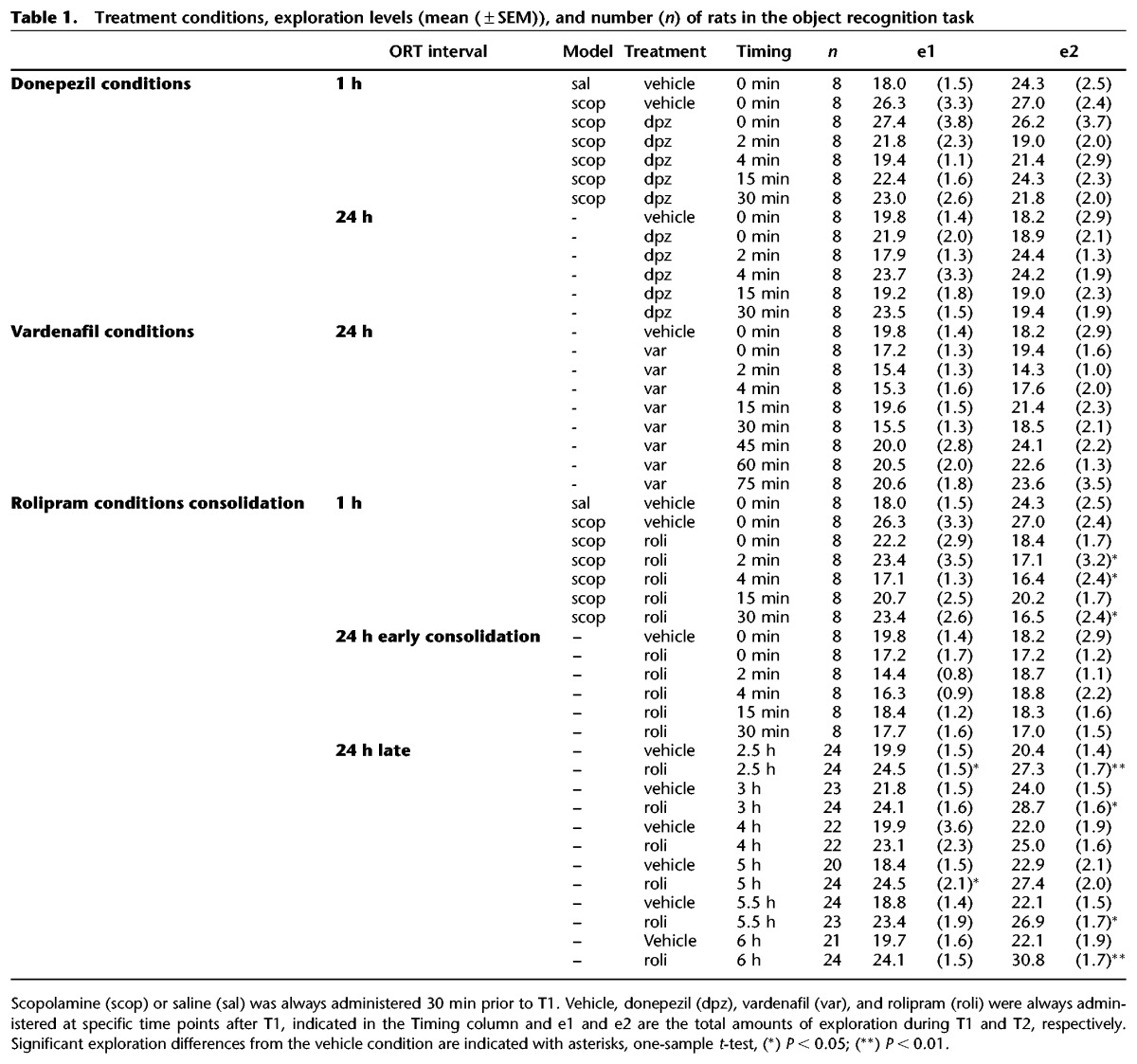
In the rolipram late consolidation experiment, independent samples t-tests were performed between the vehicle- and rolipram-treated animals at each separate injection time point, which showed that animals had object discrimination when treated with rolipram between 3 and 5.5 h after T1 (see Fig. 4).
Figure 4.
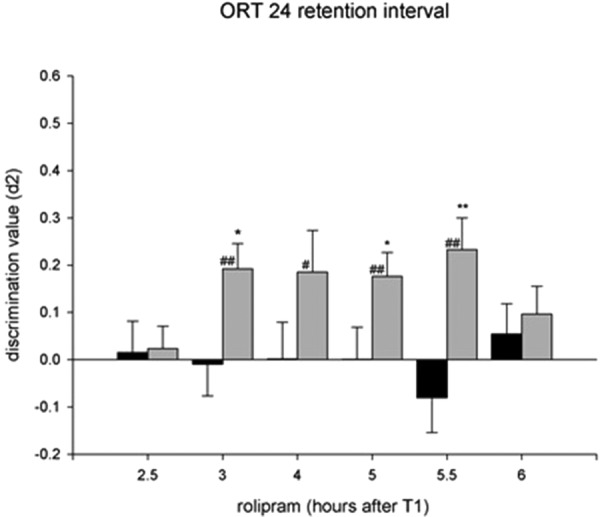
Effects of rolipram treatment on discrimination performance in an object recognition task using a 24-h retention interval. Asterisks indicate a difference from vehicle-treated animals, independent samples t-tests; (*) P < 0.05; (**) P < 0.01 and differences from zero are indicated with hashes, one-sample t-test, # P < 0.05; ## P < 0.01.
Drug exposure–time relationships in rats following administration of vardenafil, rolipram, and donepezil
The results of the pharmacokinetic measurements are shown in Tables 2 and 3. Donepezil levels more than lower limit of quantification (LLOQ) in the brain at 4 min after p.o. injection. The unbound brain/plasma ratio varied between 16.66 (10 min) to 6.40 (60 min) and unbound brain donepezil levels >IC50 were detected 10–60 min after oral administration (see Table 2).
Table 2.
Pharmacokinetic measurements of donepezil in the rat after oral administration
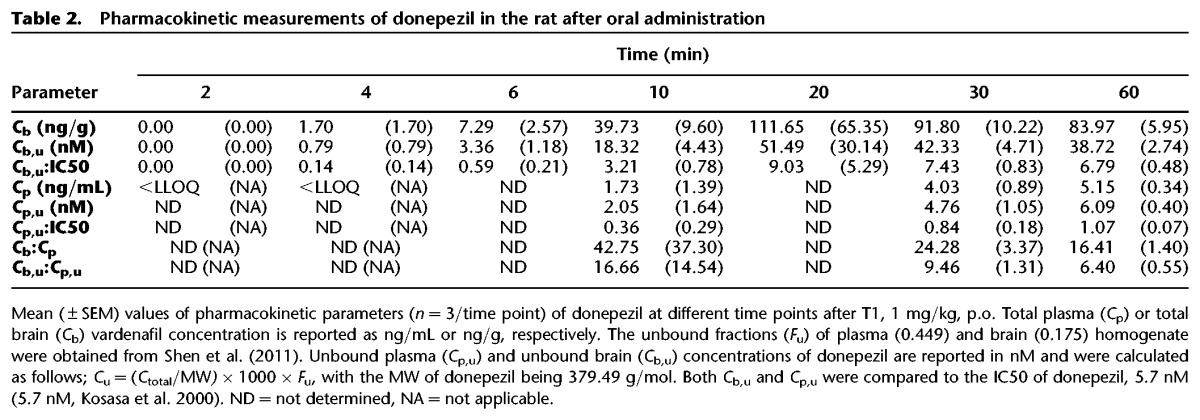
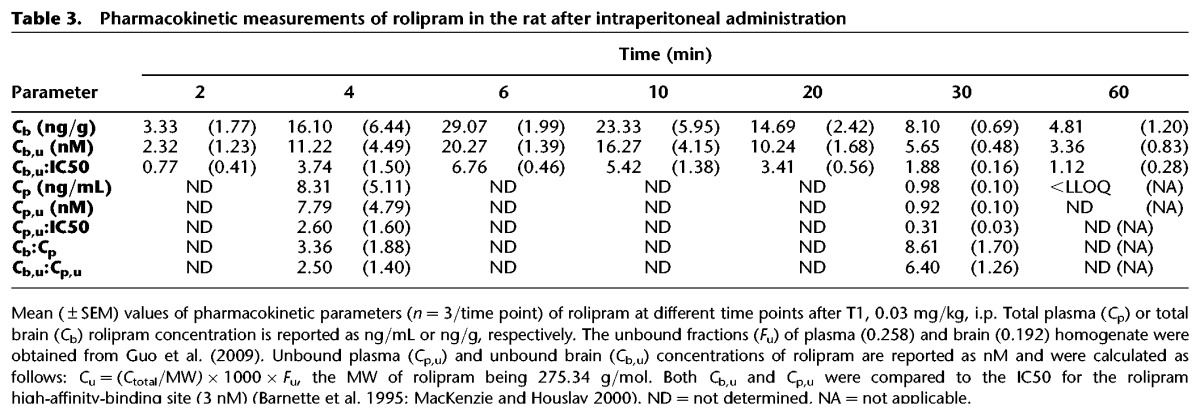
Table 3.
Pharmacokinetic measurements of rolipram in the rat after intraperitoneal administration
In a previous study we showed that vardenafil levels reached values >LLOQ in both plasma and brain, 4 min after p.o. injection (Akkerman et al. 2015). The reported unbound brain/plasma ratio varied between 1.61 (4 min) to 0.45 (20 min) and unbound brain and plasma levels of vardenafil reached IC50 values around 10 min after administration and remained >IC50 until 60 min after administration.
Rolipram was given i.p. and reached >LLOQ levels in the brain at 2 min after injection. The unbound brain/plasma ratio varied between 2.50 (4 min) to 6.40 (30 min) and unbound brain and levels of rolipram reached values >IC50 at 4 min after administration where they remained until 60 min after administration (see Table 3).
Discussion
In this study we examined the effects of different drugs that previously have been characterized as having an effect on acquisition, early consolidation, or late consolidation, i.e., donepezil, vardenafil, and rolipram, respectively. Here we examined the effects of these drugs in a model of scopolamine-induced acquisition impairment and a model of natural forgetting using a 24-h retention interval. All drugs were already detectable in the brain 2–4 min after injection. Both donepezil and vardenafil reached central levels above IC50 between 10 and 60 min after injection, while rolipram already reached IC50 within 4 min. In accordance with one of our previous studies (Akkerman et al. 2015), we found that all drugs were able to reverse a scopolamine-induced short-term memory deficit when injected within a window of 2 min post-T1. These current findings together with a previous study (Akkerman et al. 2015) then indicate that donepezil, vardenafil, and rolipram reverse the scopolamine-induced short-term memory deficit. These effects are assumed to be related to acquisition processes, which appear to linger on for several minutes after the acquisition trial. Donepezil and rolipram also prevented normal forgetting when injected within 2 min after the acquisition trial. These data further suggest an effect on acquisition processes. In the 24-h delay condition, the treatment window of vardenafil lasted up to 45 min, indicating that this represents the window for cGMP-mediated early consolidation processes. Finally, the data of rolipram in the 24-h delay interval indicate that cAMP-mediated late consolidation processes take place between 3 and 5.5 h after acquisition. Taken together, our study suggests that ACh, cGMP, and cAMP are all involved in acquisition processes and that cGMP and cAMP are also involved in early and late consolidation processes, respectively.
ACh is considered to be an important neurotransmitter for memory function. However, the specific role of ACh in memory formation is still poorly understood, possibly because different stages of memory are differentially affected by it (Micheau and Marighetto 2011). The biphasic hypothesis of cholinergic modulation of memory processes states that ACh enhances the level of afferent input of new information to the cortex via its nicotinic receptors (Hasselmo 2006). This way, high levels of ACh would lead to efficient acquisition and encoding of new information (Rasch et al. 2006).
It has been shown that post-learning blockage of muscarinic or nicotinic receptors alone does not impair consolidation processes (Rush 1988; Spangler et al. 1988; Anagnostaras et al. 1999; Miranda et al. 2003; Rasch et al. 2006;). In addition, the retention interval we used was 1 h, which is too short to implicate long-term memory consolidation processes. Therefore, we assume that, in our study, the muscarinic antagonist scopolamine-induced memory impairment was acquisition related and not due to deficient consolidation.
Surprisingly, the results of the present study showed that, in a 24-h retention interval, donepezil prevented natural forgetting of object information in rats when administered within 2 min after the T1. However, when injected after 4, 15, or 30 min after T1, donepezil was no longer effective. Donepezil showed an exactly identical effective window in the scopolamine model. These findings are in accordance with findings from other one-trial recognition studies testing acetylcholinesterase inhibitors (AChE-Is) using 24-h delay intervals. Lamirault et al. (2003) found that AChE-I (-)-9-dehydrogalanthaminium bromide successfully improved object and place memory, when injected 20 min before or directly after the acquisition phase. Also another AChE-I, tacrine, was shown to be effective, when administered directly after learning, in a social recognition task (Gheusi et al. 1994). These memory-enhancing effects of donepezil on long-term memory are unlikely to be consolidation related. On the contrary, according to the biphasic hypothesis, the increase in cholinergic activity would lead to a reduction in consolidation efficiency (Hasselmo et al. 2004; Hasselmo and Giocomo 2006; Rasch et al. 2006). Our behavioral findings show that the window during which cholinergic intervention is able to still influence what is probably acquisition lasts for at least 12 min (taking into account the injection interval and brain penetration) after a learning trial.
In the 24-h retention sessions, vardenafil improved object memory when injected up to 45 min after the learning trial. This finding is in line with our previous studies in which we found an effect when vardenafil was administered immediately after the acquisition trial but not when injected 1 h after the acquisition trial (Prickaerts et al. 2002; Rutten et al. 2007). The present data provide further support that enhancing effects of vardenafil on memory consolidation processes can be found up to 50 min after T1 (taking into account the injection interval and brain penetration). The effect of vardenafil on consolidation processes is likely to be mediated via glutamatergic stimulation of post-synaptic GMP/PKG signaling (Lu et al. 1999; Bollen et al. 2014).
In contrast to the current 24-h retention experiment, vardenafil could only reverse a scopolamine-induced acquisition deficit when injected within 2 min after the learning trial (see also Akkerman et al. 2015). This effective window shows surprising similarities to our current findings with donepezil and may suggest that the effect of vardenafil on long-term memory is partly mediated through acquisition processes when treatment is performed shortly after the acquisition trial. Such an effect on acquisition might be explained by an increased release of neurotransmitters, including ACh and glutamate, as a results of presynaptic stimulation of cGMP/PKG signaling by vardenafil (Arancio et al. 1995).
Surprisingly, the results obtained with rolipram treatment within the first hour after T1 were very similar to those found with donepezil; however, the effects were a bit less pronounced. Rolipram only fully reversed the scopolamine-induced deficit when injected at 2 min after T1, but an intermediate effect was found when rolipram was injected immediately after T1, that is, the d2 index of rats injected at 0 min after T1 was higher compared to zero but not compared to vehicle-treated animals after 24 h. Also in the 24-h retention interval, an intermediate effect of rolipram treatment was found within the first 2 min after T1. In previous studies we never found that rolipram was effective when injected directly after T1 using a 24-h delay interval (Rutten et al. 2006, 2007, 2009; Bollen et al. 2014). However, in those studies our injection times were on average about 4–10 min after the acquisition trial due to the injection procedures including taking the rat from the apparatus and taking the injection needle(s) (see Bollen et al. 2014).
Importantly, in the present study, we took great effort and care to inject exactly at the intended time point after T1. This might explain the lack of previous findings, as the current effects of rolipram were very subtle. Rolipram increases the levels of cAMP (Barad et al. 1998; Rutten et al. 2006), and presynaptical elevation of cAMP might induce the release of neurotransmitters, including ACh (Schoffelmeer et al. 1986). Along similar lines, it has been shown that acute administration of a cAMP analog, directly after learning can improve long-term habituation in crabs (Romano et al. 1996). This effect might be related a short initial spike in cAMP levels, immediately after learning (Izquierdo et al. 2006). In our study, a reinforcement of this initial spike, by rolipram treatment, may just have been sufficient to enhance the memory trace via acquisition processes.
In agreement with our previous studies (Rutten et al. 2007; Akkerman et al. 2014) we observed that rolipram improves late consolidation processes when injected at 3 h after the acquisition trial. We now demonstrated that rolipram is exactly effective when given between 3 and 5.5 h after the acquisition trial. This late consolidation window is likely to be dependent on post-synaptic cAMP/PKA signaling (Barad et al. 1998; Rutten et al. 2006; Bollen et al. 2014) and therefore indicates that cAMP-mediated late consolidation processes take place at this time.
Remarkably, in the scopolamine deficit model all drugs were effective when injected within 2 min after T1. However, to determine the exact duration of the target processes, one has to know the pharmacokinetics of each drug, because the drugs needs some time to reach the brain instantly after peripheral administration. From the literature it is known that donepezil reaches maximum plasma levels 13 min after i.p. injection at a dose of 1 mg/kg (Nakashima et al. 2006). Maximum plasma and brain concentrations are reached simultaneously at ∼30 min after p.o. donepezil administration (1 mg/kg) with a half-life of about 1 h (Matsui et al. 1999). Thus, it seems that drug absorption following i.p. administration is about twice as rapid as p.o. administration, although the observed difference in the onset of behavioral effects is smaller (Snape et al. 1999).
Our PK measurements appear to corroborate with these reports. Although we already observed maximum concentrations in brain and plasma at 20 min post-administration, the concentrations at 30 min were almost identical to those observed by Matsui et al. (1999). It is unknown what donepezil concentration is required in the brain for memory enhancement, though it is generally accepted that an IC50 is required for a biological effect. Here we show that donepezil's Cb,u reached its IC50 within 10 min after p.o. administration and remained at or above it for up to 1 h post-dosing (Table 2). The latter is an important observation since it explains that the effect of donepezil given at 0 and 2 min after the acquisition trial cannot be explained via an effect on retrieval, as one would expect that the administration at 4, 15, or 30 min after T1 in the 1-h interval should also be effective if retrieval was involved, i.e. all these administrations would result in brain concentrations well above IC50 at the time of retrieval (T2). Also, based on the available PK, one can assume that donepezil brain concentrations after the 24-h delay interval were too low for an effect on retrieval. Additionally, the 1-h interval is too short to implicate late consolidation processes which are linked to long-term memory. Taken together these findings indicate acquisition processes as the most likely mechanism for the observed discrimination improvement.
Donepezil always improved memory when injected within 2 min after learning. Together with the IC50 this also suggests that donepezil, i.e., ACh, is active within the first 12 min after T1. Yet it cannot be ruled out that donepezil already affects acquisition at unbound concentrations below its IC50, i.e., directly after the drug becomes centrally available. As we first detected donepezil in the brain 4 min after p.o. administration, the window might actually persist for only 6 min after T1.
Vardenafil was detectable in the brain within 2 min and reaches IC50 values at 10 min after p.o. injection (see Table 3). Also based on PK measurements and behavioral data in our previous study (Akkerman et al. 2015), we argue that vardenafil is likely to be involved in acquisition processes and that these processes could extend at least 4–6 min after T1. This acquisition window matches closely with the 6 min window found for donepezil. In the current study, we further show that, when memory is not impaired by scopolamine, vardenafil prevented natural forgetting when injected up to 45 min after T1, implying that the cGMP/PKG pathway is involved in both acquisition and early consolidation processes.
Rolipram has been reported to reach maximum plasma levels 15 min after oral administration (Krause and Kuhne 1988). In our study rolipram was administered i.p., and is therefore likely to be more rapidly absorbed into the bloodstream. Our PK measurements showed that rolipram is present in the brain at 2 min after injection and unbound rolipram reached the IC50 concentration in the brain within 4 min after injection. The maximum concentration was reached at 6 min and returned to IC50 at 1 h after injection. Combined with the behavioral data, it can be argued that rolipram may be actually active up to 4–6 min after learning. Considering our finding in the scopolamine model, it is unlikely that retrieval processes were affected by rolipram because, like with donepezil, every administration time point resulted in a brain concentration above IC50 at the time of retrieval (T2). Furthermore, the half-life of rolipram is too short for any effective concentrations at the time of retrieval after the 24-h retetention interval. As the 1-h interval is too short to accommodate consolidation processes, these findings implicate that PDE4 is involved in acquisition processes and again, these seem to extend for 4–6 min after T1.
Our findings on the windows of effect of donepezil, vardenafil and rolipram suggest a temporal overlap, of at least 4–6 min, between the acquisition and early consolidation phases of memory. This window might represent a transition between acquisition processes and the consolidation stage of memory. The animals were unable to acquire object information after T1, since they have no access to the objects anymore. Also, as described above, it is unlikely that donepezil enhances consolidation. Therefore, it is possible that there are encoding processes that continue for some time after stimulus exposure. Encoding refers to the formation of relations between events in episodic memory, based on many kinds of information, such as temporal and categorical (Lepage et al. 2000). It also entails novelty/familiarity evaluation (Habib et al. 2003), and involves transperceptual processes operate beyond perceptual processes (Lepage et al. 2001).
In our experiment all drugs had a remarkably similar effective window of 2 min post-T1, during which they were capable of restoring the scopolamine memory deficit. Combined with our PK measurements, these data suggest that acquisition processes continue for 4–6 min after T1. Vardenafil prevented natural forgetting via cGMP/PKG early consolidation processes when injected within 0–45 min after T1. This shows that there is a 4–6 min window of overlap between acquisition and consolidation processes, during which the transition from acquisition into early consolidation processes might take place. Furthermore, our data indicate that cAMP/PKA signaling is crucially involved in late consolidation processes between 3 and 5.5 h after T1. It would be interesting to identify pathways that are active in-between 45 min and 3 h after T1, which might link these cGMP and cAMP signaling pathways and investigate these in a similar fashion. Knowing the complete cascades underlying long-term memory formation may give us new insight and strategies to effectively treat cognitive decline.
Materials and Methods
Animals
All experimental procedures were approved by the local ethical committee of the Maastricht University for animal experiments according to governmental guidelines. Forty-eight 5-mo-old male Wistar rats (Charles River, The Netherlands) were used for the behavioral studies and 42 of these rats were used for the pharmacokinetics study (6 animals were not used). The animals were housed individually in standard Makrolon Type III cages on sawdust bedding in an air-conditioned room (about 20°C). They were kept under a reversed 12/12-h light–dark cycle (lights on from 6 p.m. to 6 a.m.). Food and water were provided at libitum: water was acidified (pH ∼3) to prevent bacterial infections and refreshed every week. Testing was done in the same room as where the animals were housed. A radio played softly, providing background noise 24 h a day, also during testing.
Treatment
Each compound was freshly dissolved on every experimental day. Donepezil (1 mg/kg; generously given by Solvay Pharmaceuticals, Weesp, The Netherlands) was dissolved in saline and administered p.o., the injection volume was 1 mL/kg. Vardenafil (1 mg/kg; kindly donated by BAYER AG, Wuppertal, Germany) was dissolved in a vehicle containing 98% tylose (methyl-cellulose) solution (1%) and 2% tween 80, and was given orally (p.o.) at an injection volume of 1 mL/kg. The same vehicle was used for dissolving rolipram, which was given i.p. (1 mL/kg). For rolipram (Sigma-Aldrich, Zwijndrecht, the Netherlands), a dose of 0.03 mg/kg was used in the 24-h retention interval sessions and 0.1 mg/kg in the 1-h retention sessions in combination with scopolamine. A fixed dose of 0.1 mg/kg scopolamine Hbr (Sigma-Aldrich) was used as an acquisition-deficit model, and was also dissolved in saline. Scopolamine was administered intraperitoneally (i.p.) at a volume of 1 mL/kg. Doses, vehicles, and administration routes were determined based on earlier studies using these drugs (Prickaerts et al. 2005; Rutten et al. 2006, 2007).
Object recognition task
We used the ORT as described in previous studies (Prickaerts et al. 2002). Briefly, the apparatus consisted of a circular arena, 83 cm in diameter. Half of the 40 cm high wall was made of gray polyvinyl chloride (RAL 7035), and the other half of transparent polyvinyl chloride. Two objects were placed at symmetrical positions about 10 cm away from the gray wall. Four different sets of objects were used, and the objects could not be displaced by the animals.
A test session comprised two trials. During the first trial (T1), the apparatus contained two identical objects (samples). After the first 3 min exploration period, the rat was put back in its home cage. Subsequently, after a delay interval of 1 or 24 h, the rat was put back in the apparatus for the second trial (T2) that also lasted 3 min. In T2, two dissimilar objects, a familiar one (the sample) and a new one, were used. The times spent exploring each object during T1 and T2 were recorded manually with specially developed software and stored on a personal computer. The experimenter who scored (live scoring) the behavior was blind toward the treatment conditions of the rats.
Exploration was defined as follows: directing the nose toward the object at a distance of no more than 2 cm and/or touching the object with the nose. Leaning or climbing on objects was not considered to be exploration. Each object was available in triplicate, so none of the two objects from the first trial had to be used as the familiar object in the second trial. In addition, all combinations and locations of objects were used in a balanced manner to reduce potential biases due to preferences for particular locations or objects.
Experimental procedure and treatment conditions
First, the animals were adapted to the testing procedure and drug administration procedures. During this training phase, the rats were submitted to one 3 min habituation trial in the empty arena on 2 consecutive days. One day later, a 1-h interval training session (comprising two 3-min trials, i.e., T1 and T2) was performed in which the animals are not subjected to any injections. During this and following sessions, two different objects were present. This was followed by a second training session in which a 24-h interval test was applied, also without injecting the animals. On a subsequent day, animals were familiarized to the administration procedure via saline injections. Animals received two saline injections, one i.p. injection and one p.o. (gavage) injection, both 1 mL/kg. Training was completed with a 1-h interval test, together with an i.p. injection, 30 min before T1 and a p.o. injection, directly after T1. On both occasions saline was administered (1 mL/kg). After this, drug testing started.
To specifically investigate the effects of donepezil, vardenafil, and rolipram on acquisition processes, a 1-h delay interval was used in combination with a scopolamine-induced memory deficit. One hour intervals were tested three times a week, on Monday, Wednesday, and Friday, and Tuesday and Thursday were used as washout days.
Since we expected the drug treatments to improve memory consolidation performance, we also tested each drug in a 24-h delay interval. Normally, rats show no more discrimination between the objects after such a delay (Akkerman et al. 2012). Each week two test sessions were performed, one session on Monday (T1) and Tuesday (T2) and the other one comprised Thursday (T1) and Friday (T2).
Testing conditions
We used 24 rats to examine drug effects in a narrow time window. Donepezil, vardenafil, and rolipram were injected at several specific time points, starting directly after T1. At 1-h delay intervals, these drugs were injected at 0, 2, 4, 15, or 30 min after T1, combined with scopolamine treatment at 30 min before T1. In the control conditions, animals received either scopolamine, or just saline (1 mL/kg, i.p.) 30 min before T1 in combination with the vehicle of vardenafil and rolipram (1 mL/kg, p.o.) immediately (0 min) after T1.
At 24-h delay intervals, donepezil or vardenafil was injected 0, 2, 4, 15, 30, 45, 60, or 75 min after T1. Rolipram was injected at 0, 2, 4, 15, or 30 min after T1 when tested in the 24-h delay intervals. As a control condition, the vehicle of vardenafil and rolipram was injected (1 mL/kg, p.o.) immediately (0 min) after T1. The same rats were used for each drug and interval condition (31 sessions in total). Another batch of 24 rats was used for an additional experiment to determine the effective treatment window for rolipram on late consolidation. In the late consolidation experiment, rolipram was tested when injected at 2.5, 3, 4, 5, 5.5, and 6 h after T1; each time point had its own vehicle condition.
Pharmacokinetics
Forty-two rats of the group that were used for behavioral test were used for the determination of plasma and brain concentrations over the first hour following its oral administration. Animals were sacrificed for blood and brain sampling at 2, 4, 6, 10, 20, 30, and 60 min after dosing. Twenty-one animals were used for donepezil measurements and 21 animals for rolipram (n = 3 per time point). The pharmacokinetics of vardenafil, using similar Materials and Methods, have been reported elsewhere (Akkerman et al. 2015). Blood was drawn from the saphenus vein using heparin-coated tubes, which were temporarily stored on wet ice, and immediately after blood collection the animal was decapitated. The complete brain was collected, rinsed with ice-cold saline, placed in a cup, weighed, and immediately frozen on dry ice. Plasma was isolated using centrifugation (1500g for 10 min at 4°C) and pipetted into Eppendorf vials. Plasma and brain samples were stored at −80°C until bioanalytical processing. Vardenafil quantification was performed at BioDuro (Pharmaceutical Product Development, Inc., Shanghai, PRC), rolipram and donepezil were quantified by Agilux Laboratories (Worcester, MA). For bioanalytical sample preparation, plasma was used as is, whereas brain samples were first homogenized in a fourfold volume (w/v) of water (vardenafil) or 80:20 water:acetonitrile (rolipram, donepezil). Both matrices were processed for drug quantification using the liquid–liquid extraction methodology followed by a characterized liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay. Standard curves were prepared in control matrices, an appropriate dynamic range was achieved, and instrument settings and potentials were adjusted to optimize the MS signal for vardenafil. Vardenafil data were processed using the Analyst Software, version 1.4.2 (AB Sciex Inc., Ontario, Canada), while for rolipram and donepezil data were processed using the Masslynx software with the Quanlynx application manager (Waters Ltd.). The LLOQ of vardenafil was 0.05 ng/mL for plasma and 0.02 ng/mL for brain. For rolipram and donepezil, the LLOQ was 0.5 ng/mL for plasma and 0.25 ng/mL for brain. The unbound fractions (Fu) of plasma and brain were obtained from the literature and unbound plasma (Cp,u) and unbound brain (Cb,u) concentrations were calculated as follows: Cu = (Ctotal/MW) × 1000 × Fu, and reported in nM. Additionally, plasma and brain concentrations were compared to IC50 values and the ratio between brain (Cb) and plasma (Cp) concentrations was calculated by dividing Cb by Cp.
Statistical analysis
The basic output measures in the ORT are the times spent by rats in exploring each object during T1 and T2. The time spent in exploring the two identical sample objects in T1 will be represented by “a1” and “a2”. The time spent in T2 in exploring the sample and the novel object will be represented by “a3” and “b,” respectively. The following variables were calculated: e1 = a1 + a2, e2 = a3 + b, and d2 = (b – a3)/e2. e1 and e2 are measures of the total time spent investigating both objects during T1 and T2, respectively. The d2 index is a relative measure of discrimination corrected for the level of exploration in the test trial (e2).
One-sample t-statistics were performed to assess whether d2 was different from zero, since random exploration in T2 would result in equal exploration of both objects. Between-group effects on e1, e2, and d2 measures were also assessed using one-way ANOVA. In case of significant differences, post-hoc comparisons with the control conditions were performed using Fisher LSD tests.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.040162.115.
References
- Abel T, Lattal KM. 2001. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol 11: 180–187. [DOI] [PubMed] [Google Scholar]
- Akkerman S, Blokland A, Reneerkens O, van Goethem NP, Bollen E, Gijselaers HJ. 2012. Object recognition testing: Methodological considerations on exploration and discrimination measures. Behav Brain Res 232: 335–347. [DOI] [PubMed] [Google Scholar]
- Akkerman S, Blokland A, Prickaerts J. 2014. Mind the gap: Delayed manifestation of long-term object memory improvement by phosphodiesterase inhibitors. Neurobiol Learn Mem 109: 139–143. [DOI] [PubMed] [Google Scholar]
- Akkerman S, Blokland A, van Goethem NP, Cremers P, Shaffer CL, Osgood SM, Steinbusch H, Prickaerts J. 2015. PDE5 inhibition improves acquisition processes after learning via a central mechanism. Neuropharmacology 97: 233–239. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Sage JR, Goodrich S, Fanselow MS. 1999. Scopolamine and pavlovian fear conditioning in rats: Dose-effect analysis. Neuropsychopharmacology 21: 731–744. [DOI] [PubMed] [Google Scholar]
- Arancio O, Kandel ER, Hawkins RD. 1995. Activity-dependent long-term enhancement of transmitter release by presynaptic 3′,5′-cyclic GMP in cultured hippocampal neurons. Nature 376: 74–80. [DOI] [PubMed] [Google Scholar]
- Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. 1998. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci 95: 15020–15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnette MS, Grous M, Cieslinski LB, Burman M, Christensen SB, Torphy TJ. 1995. Inhibitors of phosphodiesterase IV (PDE IV) increase acid secretion in rabbit isolated gastric glands: Correlation between function and interaction with a high-affinity rolipram binding site. J Pharmacol Exp Ther 273: 1396–1402. [PubMed] [Google Scholar]
- Bollen E, Puzzo D, Rutten K, Privitera L, De Vry J, Vanmierlo T. 2014. Improved long-term memory via enhancing cGMP-PKG signaling requires cAMP-PKA signaling. Neuropsychopharmacology 39: 2497–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devan BD, Sierra-Mercado JD, Jimenez M, Bowker JL, Duffy KB, Spangler EL. 2004. Phosphodiesterase inhibition by sildenafil citrate attenuates the learning impairment induced by blockade of cholinergic muscarinic receptors in rats. Pharmacol Biochem Behav 79: 691–699. [DOI] [PubMed] [Google Scholar]
- Devan BD, Pistell PJ, Daffin JLW, Nelson CM, Duffy KB, Bowker JL. 2007. Sildenafil citrate attenuates a complex maze impairment induced by intracerebroventricular infusion of the NOS inhibitor N[omega]-nitro-l-arginine methyl ester. Eur J Pharmacol 563: 134–140. [DOI] [PubMed] [Google Scholar]
- Domek-Lopacinska K, Strosznajder JB. 2008. The effect of selective inhibition of cyclic GMP hydrolyzing phosphodiesterases 2 and 5 on learning and memory processes and nitric oxide synthase activity in brain during aging. Brain Res 1216: 68–77. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Bluthe RM, Goodall G, Dantzer R. 1994. Ethological study of the effects of tetrahydroaminoacridine (THA) on social recognition in rats. Psychopharmacology (Berl) 114: 644–650. [DOI] [PubMed] [Google Scholar]
- Guo Q, Brady M, Gunn RN. 2009. A biomathematical modeling approach to central nervous system radioligand discovery and development. J Nucl Med 50: 1715–1723. [DOI] [PubMed] [Google Scholar]
- Habib R, McIntosh AR, Wheeler MA, Tulving E. 2003. Memory encoding and hippocampally-based novelty/familiarity discrimination networks. Neuropsychologia 41: 271–279. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. 2006. The role of acetylcholine in learning and memory. Curr Opin Neurobiol 16: 710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Giocomo LM. 2006. Cholinergic modulation of cortical function. J Mol Neurosci 30: 133–135. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, McGaughy J, Laurent Descarries KK, Mircea S. 2004. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog Brain Res 145: 207–231. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Bevilaqua LR, Rossato JI, Bonini JS, Medina JH, Cammarota M. 2006. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci 29: 496–505. [DOI] [PubMed] [Google Scholar]
- Klinkenberg I, Blokland A. 2010. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci Biobehav Rev 34: 1307–1350. [DOI] [PubMed] [Google Scholar]
- Kosasa T, Kuriya Y, Matsui K, Yamanishi Y. 2000. Inhibitory effect of orally administered donepezil hydrochloride (E2020), a novel treatment for Alzheimer's disease, on cholinesterase activity in rats. Eur J Pharmacol 389: 173–179. [DOI] [PubMed] [Google Scholar]
- Krause W, Kuhne G. 1988. Pharmacokinetics of rolipram in the rhesus and cynomolgus monkeys, the rat and the rabbit. Studies on species differences. Xenobiotica 18: 561–571. [DOI] [PubMed] [Google Scholar]
- Lamirault L, Guillou C, Thal C, Simon H. 2003. (−)-9-Dehydrogalanthaminium bromide, a new cholinesterase inhibitor, enhances place and object recognition memory in young and old rats. Neurobiol Learn Mem 80: 113–122. [DOI] [PubMed] [Google Scholar]
- Lepage M, Habib R, Cormier H, Houle S, McIntosh AR. 2000. Neural correlates of semantic associative encoding in episodic memory. Cogn Brain Res 9: 271–280. [DOI] [PubMed] [Google Scholar]
- Lepage M, McIntosh AR, Tulving E. 2001. Transperceptual encoding and retrieval processes in memory: A PET study of visual and haptic objects. Neuroimage 14: 572–584. [DOI] [PubMed] [Google Scholar]
- Lu YF, Kandel ER, Hawkins RD. 1999. Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. J Neurosci 19: 10250–10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie SJ, Houslay MD. 2000. Action of rolipram on specific PDE4 cAMP phosphodiesterase isoforms and on the phosphorylation of cAMP-response-element-binding protein (CREB) and p38 mitogen-activated protein (MAP) kinase in U937 monocytic cells. Biochem J 347: 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Mishima M, Nagai Y, Yuzuriha T, Yoshimura T. 1999. Absorption, distribution, metabolism, and excretion of donepezil (Aricept) after a single oral administration to Rat. Drug Metab Dispos 27: 1406–1414. [PubMed] [Google Scholar]
- Micheau J, Marighetto A. 2011. Acetylcholine and memory: A long, complex and chaotic but still living relationship. Behav Brain Res 221: 424–429. [DOI] [PubMed] [Google Scholar]
- Miranda MI, Ferreira G, Ramírez-Lugo L, Bermúdez-Rattoni F. 2003. Role of cholinergic system on the construction of memories: Taste memory encoding. Neurobiol Learn Mem 80: 211–222. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Itoh K, Kono M, Nakashima MN, Wada M. 2006. Determination of donepezil hydrochloride in human and rat plasma, blood and brain microdialysates by HPLC with a short C30 column. J Pharm Biomed Anal 41: 201–206. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, van Staveren WCG, Sik A, Markerink-van Ittersum M, Niewöhner U, van der Staay FJ. 2002. Effects of two selective phosphodiesterase type 5 inhibitors, sildenafil and vardenafil, on object recognition memory and hippocampal cyclic GMP levels in the rat. Neuroscience 113: 351–361. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, Sik A, van der Staay FJ, de Vente J, Blokland A. 2005. Dissociable effects of acetylcholinesterase inhibitors and phosphodiesterase type 5 inhibitors on object recognition memory: Acquisition versus consolidation. Psychopharmacology (Berl) 177: 381–390. [DOI] [PubMed] [Google Scholar]
- Rasch BH, Born J, Gais S. 2006. Combined blockade of cholinergic receptors shifts the brain from stimulus encoding to memory consolidation. J Cogn Neurosci 18: 793–802. [DOI] [PubMed] [Google Scholar]
- Romano A, Locatelli F, Delorenzi A, Pedreira ME, Maldonado H. 1996. Effects of activation and inhibition of cAMP-dependent protein kinase on long-term habituation in the crab Chasmagnathus. Brain Res 735: 131–140. [DOI] [PubMed] [Google Scholar]
- Rush DK. 1988. Scopolamine amnesia of passive avoidance: A deficit of information acquisition. Behav Neural Biol 50: 255–274. [DOI] [PubMed] [Google Scholar]
- Rutten K, Prickaerts J, Blokland A. 2006. Rolipram reverses scopolamine-induced and time-dependent memory deficits in object recognition by different mechanisms of action. Neurobiol Learn Mem 85: 132–138. [DOI] [PubMed] [Google Scholar]
- Rutten K, Prickaerts J, Hendrix M, van der Staay FJ, Sik A, Blokland A. 2007. Time-dependent involvement of cAMP and cGMP in consolidation of object memory: Studies using selective phosphodiesterase type 2, 4 and 5 inhibitors. Eur J Pharmacol 558: 107–112. [DOI] [PubMed] [Google Scholar]
- Rutten K, Van Donkelaar EL, Ferrington L, Blokland A, Bollen E, Steinbusch HW. 2009. Phosphodiesterase inhibitors enhance object memory independent of cerebral blood flow and glucose utilization in rats. Neuropsychopharmacology 34: 1914–1925. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer AN, Wardeh G, Mulder AH. 1986. Cyclic AMP facilitates the electrically evoked release of radiolabelled noradrenaline, dopamine and 5-hydroxytryptamine from rat brain slices. Naunyn Schmiedebergs Arch Pharmacol 330: 74–76. [DOI] [PubMed] [Google Scholar]
- Shen F, Smith JA, Chang R, Bourdet DL, Tsuruda PR, Obedencio GP. 2011. 5-HT(4) receptor agonist mediated enhancement of cognitive function in vivo and amyloid precursor protein processing in vitro: A pharmacodynamic and pharmacokinetic assessment. Neuropharmacology 61: 69–79. [DOI] [PubMed] [Google Scholar]
- Snape MF, Misra A, Murray TK, De Souza RJ, Williams JL, Cross AJ. 1999. A comparative study in rats of the in vitro and in vivo pharmacology of the acetylcholinesterase inhibitors tacrine, donepezil and NXX-066. Neuropharmacology 38: 181–193. [DOI] [PubMed] [Google Scholar]
- Spangler EL, Chachich ME, Ingram DK. 1988. Scopolamine in rats impairs acquisition but not retention in a 14-unit T-maze. Pharmacol Biochem Behav 30: 949–955. [DOI] [PubMed] [Google Scholar]
- van Donkelaar EL, Rutten K, Blokland A, Akkerman S, Steinbusch HWM, Prickaerts J. 2008. Phosphodiesterase 2 and 5 inhibition attenuates the object memory deficit induced by acute tryptophan depletion. Eur J Pharmacol 600: 98–104. [DOI] [PubMed] [Google Scholar]
- van Goethem NP, Rutten K, van der Staay FJ, Jans LAW, Akkerman S, Steinbusch HWM. 2012. Object recognition testing: Rodent species, strains, housing conditions, and estrous cycle. Behav Brain Res 232: 323–334. [DOI] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. 2006. Paradoxical facilitation of object recognition memory after infusion of scopolamine into perirhinal cortex: Implications for cholinergic system function. J Neurosci 26: 9520–9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. 2008. Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev 32: 1055–1070. [DOI] [PubMed] [Google Scholar]