Abstract
Introduction
Magnetic Resonance Elastography (MRE) analyzes shear waves’ movement thorough tissue to determine stiffness. In a prior study, measurements using first-generation brain MRE techniques correlated with intraoperative observations regarding overall meningioma stiffness. We evaluated the diagnostic accuracy of a higher-resolution MRE technique to preoperatively detect intratumoral variations as compared to surgeon assessment.
Methods
Fifteen meningiomas in fourteen patients underwent MRE. Tumors with regions of distinctly different stiffness were considered heterogenous. Intratumoral portions were considered hard if there was a significant area ≥ 6 kiloPascals. A 5-point scale graded intraoperative consistency. A durometer semi-quantitatively measured surgical specimen hardness. Statistics included Chi-squared, sensitivity, specificity, positive and negative predicative values (PPV and NPV), and Spearman’s rank correlation coefficient.
Results
Between MRE and surgery respectively, 9(60%) vs 7(47%) tumors were homogenous; 6(40%) vs 8(53%) tumors were heterogenous; 6(40%) vs 10(67%) tumors had hard portions; and 14(93%) vs 12(80%) tumors had soft portions. MRE sensitivity, specificity, PPV and NPV were: for heterogeneity, 75%, 100%, 100%, and 87%; for hardness, 60%, 100%, 100%, and 56%; and for softness, 100%, 33%, 86%, and 100%. Overall, 10(67%) tumors matched well with MRE and intraoperative consistency and correlated between intraoperative observations (p=0.018) and durometer readings (p=0.046). Tumor size ≤3.5 cm or vascular tumors were more likely to be inconsistent (p<0.05).
Conclusions
MRE was excellent at ruling-in heterogeneity with hard portions, but less effective in ruling-out heterogeneity and hard portions, particularly in tumors more vascular or <3.5 cm. MRE is the first technology capable of prospectively evaluating intratumoral stiffness and, with further refinement, will likely prove useful in preoperative planning.
INTRODUCTION
Since the advent of computed tomography (CT) and magnetic resonance imaging (MRI), there have been attempts to prospectively determine the intraoperative consistency of meningiomas, all of which were based on static imaging characteristics 1–7. Magnetic Resonance Elastography (MRE) is a dynamic MRI-based technique that measures the propagation of mechanically induced shear waves through a particular tissue to determine stiffness and offers a method to evaluate tissue consistency8–11. In essence, MRE “palpates” by imaging and measures a property called the shear modulus, which varies by over five orders of magnitude between normal and pathologic tissues. In comparison, the assessment of tissue by CT, MRI, and ultrasound is less than 2 orders of magnitude 11. This difference was illustrated in a prior study by Murphy et al 5 comparing MRE and a previously described technique using standard T1 and T2-weighted MRI images to determine the consistency of meningiomas 2; MRE correlated better with regard to stiffness and offered a more graded scale, distinguishing not just between “firm” and “soft” but also “intermediate”5.
As the role of endoscopic and other minimally invasive approaches for intracranial tumor resection becomes more prevalent, there is greater need for an imaging modality that can give detailed information regarding tumor consistency 12. Ostensibly, tumors that are harder would be more amenable to conventional open approaches, while softer tumors might favor minimally invasive procedures; in addition, knowing the consistency of a tumor would aid in preoperative planning regardless of the approach. However, meningiomas can have varying consistency within the tumor and a technology that could determine differences in intratumoral consistency would be ideal.
MRE is still a developing technology, especially with regards to intracranial applications, and the imaging resolution has not reached the full potential of being able to distinguish finer gradations of intratumor consistency. The purpose of this study was to evaluate whether a MRE with a higher-resolution than used by Murphy et al5 could differentiate heterogeneous intratumoral stiffness in a prospective series of meningiomas.
METHODS
After institutional review board approval, 15 meningiomas in 14 patients were prospectively evaluated by MRE prior to surgery from October 2013 to June 2014. Inclusion criteria were patients with a tumor presumed to be a meningioma > 2 cm in diameter that required preoperative imaging at our institution and were referred by the operating surgeon for MRE. Exclusion criteria were patients that had imaging from outside institutions and did not require further preoperative scans. MRE was performed with a modified single-shot spin-echo echo-planar-imaging pulse sequence on a 3T MRI system (Signa Excite, GE Healthcare, Waukesha, WI). The same MRE image acquisition was used as previously reported 5. Shear waves were introduced intracranially with a soft, pillow-like pneumatic driver positioned under the subject’s head. The resulting displacement field was acquired with: TR/TE 3600/62 ms, FOV = 24 cm, 3 x parallel imaging acceleration, 48 contiguous 3 mm thick axial slices and 8 phase offsets sampled over one period of the 60 Hz motion. The resulting images have 3 mm isotropic resolution [compared with 4 mm with first generation techniques 5] and were acquired in just under 7 minutes. The curl of the wave images and stiffness were calculated with a direction-inversion algorithm.
Tumor characteristics evaluated were intratumoral homogeneity, heterogeneity, and stiffness [shear wave speed squared in kilopascal (kPa)]. MRE measures stiffness by calculating the median stiffness in region(s)-of-interest(s) (ROI). A tumor was considered hard if it had a stiffness value greater than 6 kPa (range 0–8). If 20% of the tumor had ROI as determined by an experienced radiologist (JH) with distinctly different stiffness values, it was considered heterogenous (Figure 1), while tumors with less variability were considered homogenous (Figure 2). Magnetic Resonance Elastography values were calculated prospectively by radiologists unaware of the surgical findings. Tumor size was noted in centimeters (cm) of the largest maximal dimension. Vascular tumors were defined as having prominent intratumoral flow voids.
Figure 1.
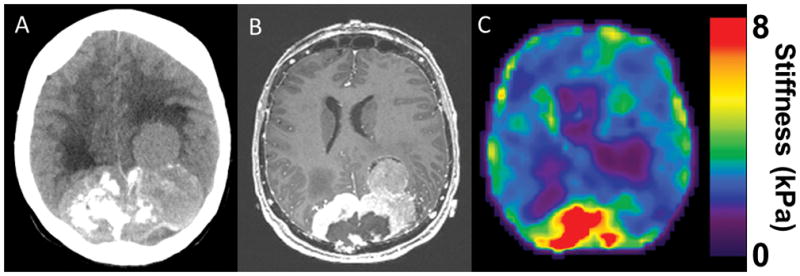
(A) CT head of a large parietooccipital parasagittal meningioma with a nodule of tumor on the left extending further anteriorly. The right side of the tumor is calcified. (B) T1 weighted MRI with contrast defines the tumor further.(C) MRE shows the tumor is heterogenous with the posterior portion being hard and the more left lateral and anterior nodule becoming progressively softer. Intraoperatively, the posterior region of the tumor had to be removed with heavy scissors, but as the dissection moved to the left and anteriorly, the tumor was easily removed with the ultrasonic aspirator at low settings.
Figure 2.
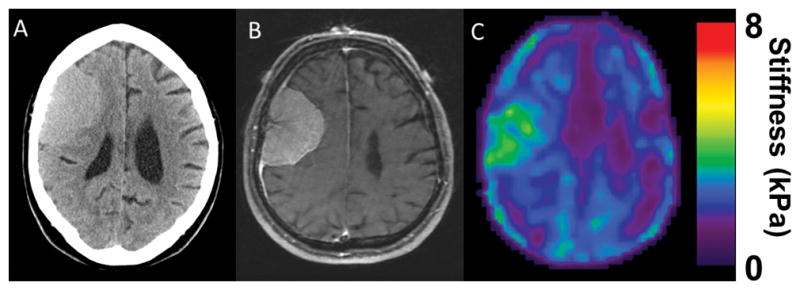
(A) CT head of an isodense right frontal convexity tumor. (B) T1 weighted MRI with contrast shows a homogenously enhancing tumor consistent with meningioma.(C) MRE shows a soft homogenous tumor. Intraoperatively, the tumor was easily removed with ultrasonic aspirator and was consistent throughout.
Surgeon impression at resection was considered the reference standard. All surgeons were experienced meningioma surgeons and familiar with the variability that might be present in tumor tissue. Participating surgeons (FM, GL, JVG, ML) blinded to the results of the MRE, reported overall as well as intratumoral consistency at the time of surgery in detailed surgical notes, which were reviewed retrospectively. The tumor was considered homogenous if it was consistent throughout and the same surgical instrument worked well for the whole tumor. The tumor was considered heterogenous if different tissue consistencies were encountered based on surgeons’ impression and required a wide-variety of instruments to remove. Surgeons’ remarked on the anatomical locations where they encountered different tissue consistencies. They also remarked on tumor vascularity. Tumor stiffness was graded on the following scale: (1) removed mostly with suction (2) removed with combination suction and ultrasonic aspirator (3) removed mostly with ultrasonic aspirator (4) difficult for ultrasonic aspirator (5) required scissors or cautery.
A Shore durometer (Type 00, model 1600, Rex Durometers, Rex Gauge Company, Buffalo Grove, IL) was used to semi-quantitatively measure the hardness of surgical specimens to verify both MRE measurements and surgeon impression. The device uses a pressor foot that gauges hardness by measuring how far the material indents the foot, with higher numbers indicating a harder material. It is a dimensionless quantity. Surgical specimens were taken from different regions of tumors. If the tumor was heterogenous, the location of that approximate anatomical location was noted for later correlation with MRE. Multiple measurements were taken and averaged from each specimen.
Chi-squared, sensitivity, specificity, positive and negative predicative values (PPV and NPV), and Spearman’s rank correlation coefficient were calculated with JMP Version 10.0 (SAS, Cary, NC) to compare surgical grading, durometer measurements, and MRE stiffness values. P-values <0.05 were considered significant.
RESULTS
Four patients were male and ten were female with an average age of 59 ± 12 years. See Table 1 for patient demographics, presentation, and tumor characteristics.
TABLE 1.
Patient demographics, presentation, and tumor characteristics
| Age | Sex | Presenting Symptoms | Tumor Location | Tumor Size (cm) |
|---|---|---|---|---|
| 65 | M | Left weakness, personality changes | Convexity | 8.0 × 6.0 × 6.0 |
| 71 | F | Visual disturbance, confusion | Falcine | 7.0 × 9.0 × 5.0 |
| 55 | F | Mild Right weakness | Foramen magnum | 3.4 × 2.6 × 3.5 |
| 28 | M | Mild Left weakness | Convexity | 5.7 × 6.5 × 5.5 |
| 50 | F | Left visual deficit | Planum | 2.2 × 2.2 × 1.5 |
| 66 | F | Personality changes | Sphenoid wing | 4.4 × 5.0 × 4.9 |
| 76 | F | Imbalance | Posterior fossa | 4.3 × 4.3 × 3.6 |
| 58 | F | Mild left hemiparesis | Falcine | 3.8 × 3.8 × 3.9 4.3 × 3.3 × 3.6 |
| 76 | M | None | Convexity | 6.4 × 5.4 × 3.6 |
| 55 | F | Left facial pain | Cerebellopontine angle | 2.5 × 2.6 × 2.6 |
| 62 | F | Cogitative difficulty, gait | Olfactory groove | 5.1 × 3.4 × 4.6 |
| 47 | F | Left hearing loss | Cerebellopontine angle | 1.5 × 2.3 × 2.2 |
| 62 | F | Gait difficulty | Tentorial | 4.9 × 4.0 × 4.7 |
| 55 | M | Headache | Tentorial | 4.7 × 4.6 × 5.6 |
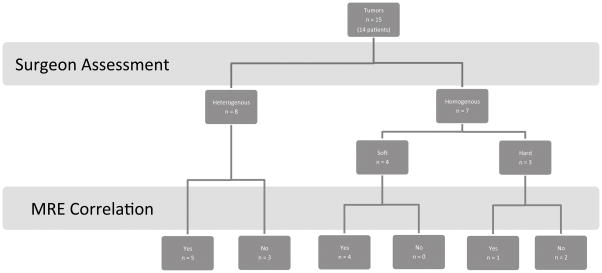
Preoperative MRE characterized 9 (60%) tumors as homogenous and 6 (40) tumors as heterogenous while surgeons determined 7(47%) tumors as homogenous and 8 (53) tumors as heterogenous. On MRE, 6 (40%) tumors were hard or had hard portions, while 14 (93%) were soft or had soft portions. On surgical evaluation, 10 (67%) tumors were hard or had hard portions, while 12 (80%) were soft or had soft portions. See Table 2for MRE sensitivity, specificity, and positive and negative predictive values.
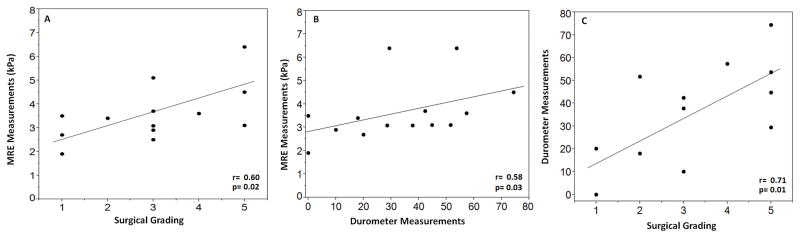
Overall, 10 (67%) tumors correlated with MRE and intraoperative findings (Figure 3). In these 10 tumors, there were 13 MRE ROIs that correlated with intraoperative observations (p=0.018) and durometer readings (p=0.046) (Figure 4, A and B). The durometer also correlated with surgeon findings (p=0.002) (Figure 4, C). Durometer readings were taken in all but one of these ten tumors.
Figure 3.
Flow diagram of MRE results compared with surgeon assessment.
Figure 4.
Graphs showing correlation of MRE measurements and the surgeons’ impression (A), MRE measurements and durometer readings (B), and durometer measurements and the surgeon’s impression (C) in the tumors that were similar between MRE and intraoperative findings. All had good correlation with p<0.005.
Of the tumors that MRE did not predict correctly, one was small and hard throughout while MRE predicated a soft, homogenous tumor. Two tumors were small, and mostly soft, but had a portion that was hard, while MRE predicted an entirely soft tumor (Figure 5, A). Two were large, vascular tumors predicted to be soft and homogenous, but were difficult to resect with everything but cautery loops and scissors (Figure 5, B). A tumor size ≤3.5 cm or very vascular tumors were more likely to be inconsistent between MRE and intraoperative findings (p<0.05).
Figure 5.
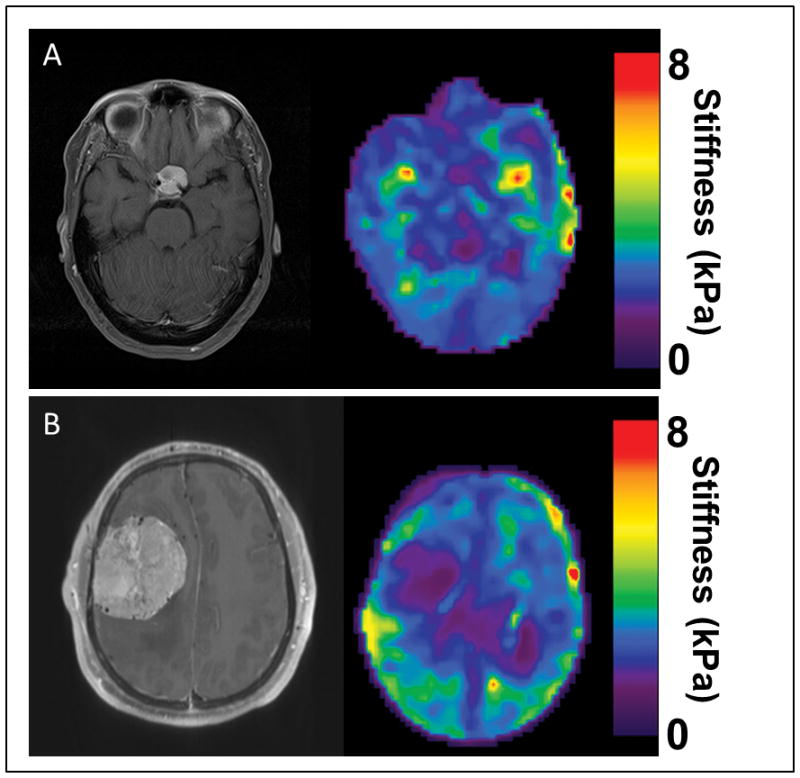
Examples of tumors that did not correlate well with MRE and surgical findings. (A). A small planum sphenoidale meningioma that measured 2.2 cm in maximum diameter. MRE showed the tumor to be homogenous and soft; intraoperatively the tumor was 70% soft, but 30% was very firm in the region along the left internal carotid artery. (B) A right convexity meningioma that measured 6.5 cm in maximum diameter. Note the flow voids within the tumor and preoperative angiography confirmed a highly vacular tumor. MRE showed the tumor to be soft and homogenous. At surgery, the tumor was consistent throughout, but required cautery to remove as it was fibrous.
DISCUSSION
In a previous study by Murphy et al 5, MRE correlated significantly with tumor stiffness more than the standard MRI method of Hoover et al 2. The MRE methodology including 4 mm isotropic resolution used in this prior study provided information regarding overall tumor consistency, but tumors can have internal variation in stiffness; a technology that can make gradations of intratumor consistency would be ideal. While the higher resolution MRE used in this study including 3 mm isotropic resolution did not correlate with highly vascular or smaller tumors, there was good correlation with the majority of meningiomas (67%).
The higher resolution MRE performed best in tumors that were hard and heterogenous (100% specificity and PPV). There was no tumor MRE predicted to have a significantly hard portion or have different areas of consistency in which this was not found intraoperatively. In other words, MRE performed well when ruling-in hardness and heterogeneity. It did not do as well ruling-in homogeneity (78% specificity and 75% PPV) and soft consistency (33% specificity and 86% positive predictive value), and ruling-out hardness (60% sensitivity).
In the prior study by Murphy et al 5, the most outlying tumor predicted to be firm but found to be soft at surgery had a firm basilar attachment, which was thought to explain the difference between surgery and MRE. This finding maybe a result of the lower spatial resolution with the immovable dural attachment simulating a stiffer tissue as occurred with the tumor in Figure 5A. Interestingly, most differences between surgery and MRE in this study were tumors predicted as soft and found to have hard portions intraoperatively. In vascular tumors, this difference is likely because of the difficulty that vascular meningiomas pose in resection. While vessels are not suckable and make resection difficult, they may be flexible and lead to a softer appearance on MRE. The other tumors that did not correlate well with MRE were small and noted to be mostly soft but had a hard portion encountered at surgery. In these, it is likely that the current MRE was not of high enough resolution to pick up this difference. It is anticipated that further technical MRE improvements including even higher spatial resolution will allow detection of smaller zones of varied stiffness within tumors.
Only one technology to date, ultrasound elastography, has reported on being able to detect intratumoral stiffness 13. This technology can only be used once the skull is removed at surgery, and has no capability to assist in preoperative planning. To date, MRE is the only technology capable of preoperatively evaluating intratumoral stiffness.
With further study, MRE would likely have a role in preoperative planning, specifically in determining endoscopic versus open approaches. In addition, as calcification and necrosis appear similar on MRI, MRE would be a means of distinguishing between the two (see Figure 1). We are also currently investigating a modality of MRE called slip-interface imaging that has the potential to assess tumor adherence to brain.
A weakness of this study is that different surgeons resected the meningiomas so grading of consistency could be different between each. However, durometer readings correlated well between MRE and surgical observations, providing evidence that the surgical observations were fairly consistent. Another weakness is that MRE measurements and durometer readings were only approximated in regard to intratumoral location based on surgeon localization. The surgeons involved were all experienced meningioma surgeons and the ROIs were large enough that more precise localization using image guidance was not necessary.
CONCLUSION
Currently, no imaging technology other than MRE is capable of prospectively predicting intratumoral consistency. The higher resolution MRE utilized in this study was able to preoperatively determine intratumoral differences in consistency in two-thirds of the meningiomas. It performed best in larger tumors that were not overly vascular. With further improvements including even higher spatial resolution, MRE has the potential to become a valuable imaging modality in preoperative planning in meningioma surgery.
TABLE 2.
Values for MRE Measurements Compared to Surgical Grading
| Heterogeneity | Homogeneity | Hardness | Softness | |
|---|---|---|---|---|
| Sensitivity | 75% | 100% | 60% | 100% |
| Specificity | 100% | 75% | 100% | 33% |
| PPV | 100% | 78% | 100% | 86% |
| NPV | 78% | 100% | 56% | 100% |
PPV = positive predictive value, NPV = negative predictive value
References
- 1.Carpeggiani P, Crisi G, Trevisan C. MRI of intracranial meningiomas: correlations with histology and physical consistency. Neuroradiology. 1993;35(7):532–536. doi: 10.1007/BF00588715. [DOI] [PubMed] [Google Scholar]
- 2.Hoover JM, Morris JM, Meyer FB. Use of preoperative magnetic resonance imaging T1 and T2 sequences to determine intraoperative meningioma consistency. Surg Neurol Int. 2011;2:142. doi: 10.4103/2152-7806.85983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kashimura H, Inoue T, Ogasawara K, et al. Prediction of meningioma consistency using fractional anisotropy value measured by magnetic resonance imaging. J Neurosurg. 2007 Oct;107(4):784–787. doi: 10.3171/JNS-07/10/0784. [DOI] [PubMed] [Google Scholar]
- 4.Kendall B, Pullicino P. Comparison of consistency of meningiomas and CT appearances. Neuroradiology. 1979 Oct 31;18(4):173–176. doi: 10.1007/BF00345721. [DOI] [PubMed] [Google Scholar]
- 5.Murphy MC, Huston J, Glaser KJ, et al. Preoperative assessment of meningioma stiffness using magnetic resonance elastography. J Neurosurg. 2013 Mar;118(3):643–648. doi: 10.3171/2012.9.JNS12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki Y, Sugimoto T, Shibuya M, Sugita K, Patel SJ. Meningiomas: correlation between MRI characteristics and operative findings including consistency. Acta Neurochir (Wien) 1994;129(1–2):39–46. doi: 10.1007/BF01400871. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi N, Kawase T, Sagoh M, Ohira T, Shiga H, Toya S. Prediction of consistency of meningiomas with preoperative magnetic resonance imaging. Surg Neurol. 1997 Dec;48(6):579–583. doi: 10.1016/s0090-3019(96)00439-9. [DOI] [PubMed] [Google Scholar]
- 8.Green MA, Bilston LE, Sinkus R. In vivo brain viscoelastic properties measured by magnetic resonance elastography. NMR Biomed. 2008 Aug;21(7):755–764. doi: 10.1002/nbm.1254. [DOI] [PubMed] [Google Scholar]
- 9.Kruse SA, Rose GH, Glaser KJ, et al. Magnetic resonance elastography of the brain. Neuroimage. 2008 Jan 1;39(1):231–237. doi: 10.1016/j.neuroimage.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sack I, Beierbach B, Hamhaber U, Klatt D, Braun J. Non-invasive measurement of brain viscoelasticity using magnetic resonance elastography. NMR Biomed. 2008 Mar;21(3):265–271. doi: 10.1002/nbm.1189. [DOI] [PubMed] [Google Scholar]
- 11.Mariappan YK, Glaser KJ, Ehman RL. Magnetic resonance elastography: a review. Clin Anat. 2010 Jul;23(5):497–511. doi: 10.1002/ca.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zada G, Yashar P, Robison A, et al. A proposed grading system for standardizing tumor consistency of intracranial meningiomas. Neurosurgical focus. 2013 Dec;35(6):E1. doi: 10.3171/2013.8.FOCUS13274. [DOI] [PubMed] [Google Scholar]
- 13.Chakraborty A, Bamber JC, Dorward NL. Slip elastography: a novel method for visualising and characterizing adherence between two surfaces in contact. Ultrasonics. 2012 Mar;52(3):364–376. doi: 10.1016/j.ultras.2011.07.001. [DOI] [PubMed] [Google Scholar]