Insight from Sachin Gadani (left) and Jonathan Kipnis (right)
In this issue of JEM, Xi et al. describe how inflammation is initiated in the retina, studying the alarmin IL-33. Expressed in healthy tissues and released in conditions of cell damage or stress, alarmins include molecules such as ATP, HMGB1, IL-1α, and IL-33. IL-33 is a nuclear protein highly expressed in skin and lung epithelial cells, CNS oligodendrocytes, and elsewhere, and is typically released by necrotic cell death to “sound the alarm” for immune cells. Interestingly, its expression in the CNS is among the highest of any tissues, although its role there has only recently begun to be studied. IL-33 is known to influence outcome of EAE, has effects on pain perception, and we and others have demonstrated its importance after traumatic CNS injury.
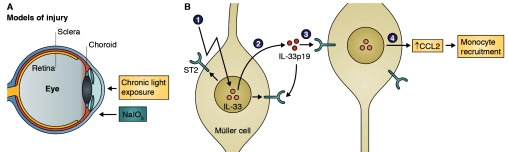
After retinal injury or stress, Müller cells produce the alarmin IL-33, leading to autocrine stimulation and monocyte recruitment through CCL2 secretion.
The current work sheds light on IL-33 in the eye, addressing the cellular localization and function of released IL-33 after retinal injury or stress. The authors show that after stressful stimuli, Müller cells release IL-33, which activates them in an autocrine manner and leads to CCL2 expression and subsequent macrophage recruitment.
IL-33 is classically thought to be released by necrotic cell death, in part because its lack of a secretory signal peptide, but numerous recent studies are challenging this notion. Contributing to this evidence, the authors demonstrate that Müller cells stressed in vitro release IL-33 without death. IL-33 is released by Müller cells as a 19-kD cleavage product, detectable by Western blot in both the nuclear and cytoplasmic fractions after cell stress.
Though expressed widely, the highest producers of IL-33 are in the skin, lung, and CNS. Skin and lung—barrier tissues, with the frequent threat of traumatic injury—must express alarmins to help rapidly mobilize a response. But why is IL-33 expressed so highly in the CNS? IL-33 presumably acts as an activator of CNS inflammation, but from an evolutionary perspective, it is hard to believe that any trait would develop and remain only for benefit during overt CNS trauma. Perhaps the real role for IL-33 is in more subtle and common CNS insults. For example, the retina is frequently exposed to light, which could cause frequent but minor cellular damage. Given the ability for Müller cells to secrete IL-33 without dying, the role of IL-33 in the healthy eye may be to balance the minor cell damage induced upon exposure to high intensity light. Alternatively, IL-33 in the CNS could have a novel function to that in the periphery. The CNS frequently reuses “immune” molecules for other purposes, such as MHC1 in synaptic plasticity or complement in synaptic pruning. Previous discoveries of context-dependent functions for “immune” molecules in the brain teach us to expect the unexpected, and we must remain open minded when studying IL-33, or any other immune molecule, in the CNS.
References
- Xi, H., et al. 2016. J. Exp. Med. 10.1084/jem.20150894 [DOI] [Google Scholar]