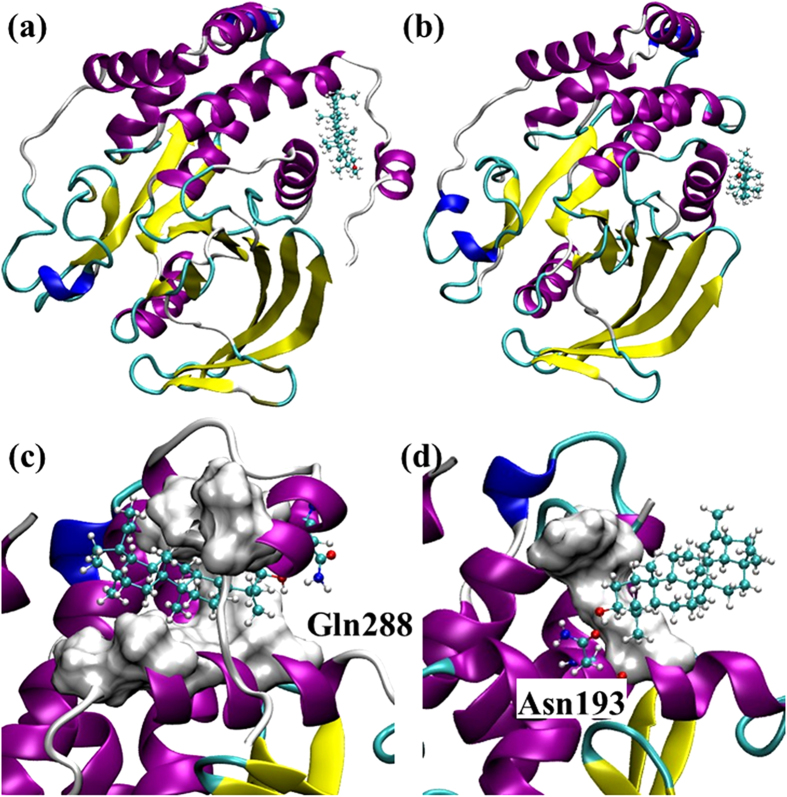
Figure 4.
The top-ranked binding poses of lupeol to the allosteric site of PTP1B299 (a) and PTP1B282 (b). Detailed interactions revealed by the molecular dynamics simulations for lupeol and PTP1B are shown in (c) and (d). The hydrophobic residues involved in the binding in PTP1B299 (Ala189, Leu192, Phe196, Phe280, Trp291, and Leu294) are shown in Surf. The polar residue involved in the hydrogen bonding Gln288 is shown in CPK (c). The hydrophobic residues involved in the binding in PTP1B282 (Phe196 and Phe280) are shown in Surf while the polar residue involved in the hydrogen bonding in Asn193 is shown in CPK (d).