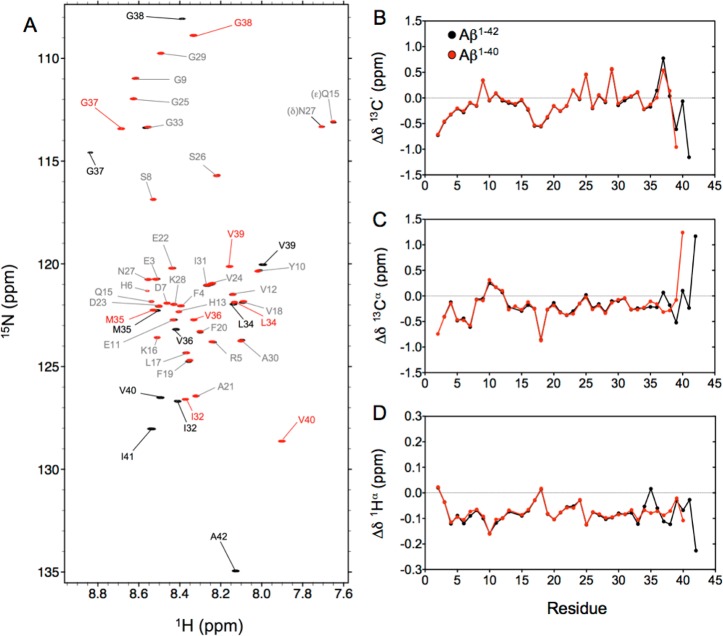
Figure 1.
(A) Overlay of the 15N–1H HSQC spectra recorded at 800 MHz for monomeric Aβ1–40 (red) and Aβ1–42 (black) peptides at 277 K. Assignments of the backbone amide cross-peaks are colored gray for residues with nearly identical chemical shifts in the two peptides, while labels in red and black (for Aβ1–40 and Aβ1–42, respectively) correspond to residues with significantly different chemical shifts in the two peptides. Secondary chemical shifts for (B) 13C′, (C) 13Cα, and (D) 1Hα nuclei of Aβ1–40 (red) and Aβ1–42 (black) were derived using random coil values and correction factors of Poulsen and co-workers.36,37