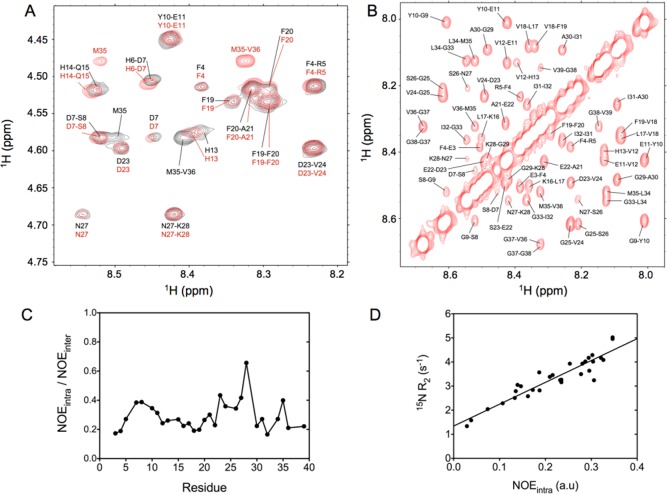
Figure 4.
Nuclear Overhauser data recorded for the Aβ peptides. (A) Expanded region of the 2D NOESY spectrum recorded at 900 MHz for the Aβ1–40 (red) and Aβ1–42 (black) peptides at 277 K. The cross-peaks correspond to sequential and intraresidue interactions between the 1HN and 1Hα protons. Significant chemical shift differences between Aβ1–40 and Aβ1–42 are seen in this region for the intraresidue HN–Hα(i,i) M35 and sequential Hα–HN(i,i+1) M35-V36 cross-peaks. (B) Expanded region of the 2D projection from the 3D NOESY-HSQC spectrum recorded at 900 MHz for Aβ1–40, showing the HN–HN region. (C) Ratio between the intraresidue daN(i,i) NOE intensity and the sequential Hα–HN(i–1,i) NOE measured for Aβ1–40 and reported as a function of residue number. (D) Correlation between the intraresidue daN(i,i) NOE intensity and the 15N transverse relaxation rate measured at a 1H frequency of 600 MHz.