Abstract
Cardiac resynchronisation therapy (CRT) is a recognised therapy for the management of severe left ventricular dysfunction, advanced congestive cardiac failure (NYHA III or IV), ventricular dyssynchrony (either broad LBBB or mechanical dyssynchrony on echocardiography) and failure of optimal medical therapy to achieve improvement in clinical status. Upgrading right ventricular pacemakers or defibrillators to biventricular devices is common and we describe here, 2 such cases of biventricular upgrade with blocked venous access on the ipsilateral side and successful placement of left ventricular leads following pre-sternal tunnelling from the contralateral side.
Keywords: Implantable cardioverter defibrillators, Cardiac resynchronisation therapy, Transvenous leads, Tunnelled lines, Blocked veins, Device upgrade
Case series
Cardiac resynchronisation therapy (CRT) is a recognised therapy for the management of severe left ventricular dysfunction, advanced congestive cardiac failure (NYHA III or IV), ventricular dyssynchrony (either broad LBBB or mechanical dyssynchrony on echocardiography) and failure of optimal medical therapy to achieve improvement in clinical status [1]. Upgrading right ventricular pacemakers or defibrillators to biventricular devices is common practice and we describe here, 2 such cases of biventricular upgrade with blocked venous access on the ipsilateral side and successful placement of left ventricular leads following pre-sternal tunnelling from the contralateral side.
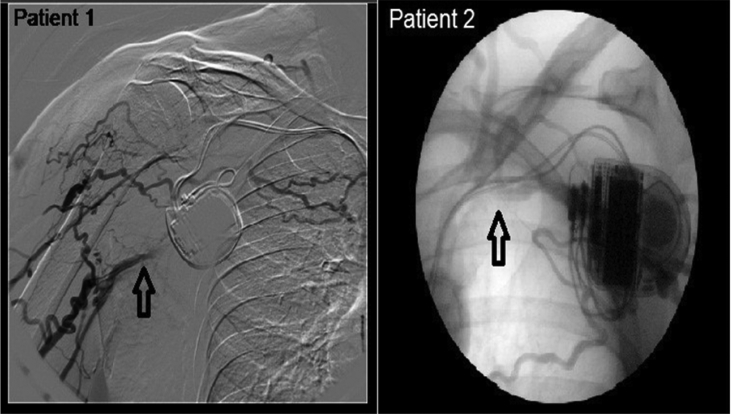
Our first patient was a 65 year old gentleman with a background of ischaemic cardiomyopathy and percutaneous coronary intervention (PCI) to his left anterior descending artery in 2006. A secondary prevention implantable cardioverter defibrillator (ICD) (St Jude Atlas DR) was inserted on the left side in 2004 for monomorphic ventricular tachycardia (VT). He required subsequent extraction due to pocket erosion and a new system was implanted on the right side. His clinical status deteriorated in the following years with NYHA class III heart failure, severe LV dysfunction (LVEF 30%) and broad LBBB (130 ms) despite optimal medical treatment and the decision was taken to upgrade his ICD to CRT-D. Given his previous device procedures, we undertook contrast venography to study the upper limb venous anatomy which confirmed occlusion of the right subclavian vein (Fig. 1).
Fig. 1.
Venogram showing blocked subclavian vein in patient 1 and patient 2.
The second patient was a 78 year old gentleman with ischaemic cardiomyopathy and LVEF of 18%. His background included moderate-severe COPD, multi-nodular goitre and post-vagotomy dumping syndrome. He had a primary prevention ICD (Medtronic Marquis 7274 device with OptiVol Sensor) and his clinical status had deteriorated over the years necessitating upgrade to CRT-D. Despite optimal medical therapy, he had NYHA class III heart failure, broad QRS duration (150 ms) and his device had detected an increase in the intrathroacic pressure (Optivol fluid index >200 and thoracic impedance <70 Ω) supporting the clinical diagnosis of deteriorating heart failure. Venogram through the left anti-cubital vein showed a short occlusion of the left subclavian vein (Fig. 1). The procedure was initially attempted on the left, with successful cannulation of the left subclavian vein but unable to advance the guidewire to the superior vena cava (SVC). The procedure was abandoned and CRT-D implantation was performed on the right.
Both procedures were performed under general anaesthesia. For the first patient with blocked right subclavian vein, a left extra-thoracic subclavian puncture was made under fluoroscopic guidance and a small pocket was formed in the prepectoral area. The coronary sinus (CS) was cannulated and a Starfix LV lead was placed in mid-position of a large anterolateral vein with satisfactory parameters (R wave 11.1 mV, impedance 556 Ω and threshold 0.8 V @ 0.4 ms). For the second patient, left extra-thoracic subclavian vein was used. The CS was cannulated with a Multipurpose catheter and a Starfix LV lead was placed in the middle branch of the three anterolateral branches with satisfactory position and lead parameters (threshold 0.7 V @ 0.5 ms, impedance 840 Ω and R wave of 11.2 mV).
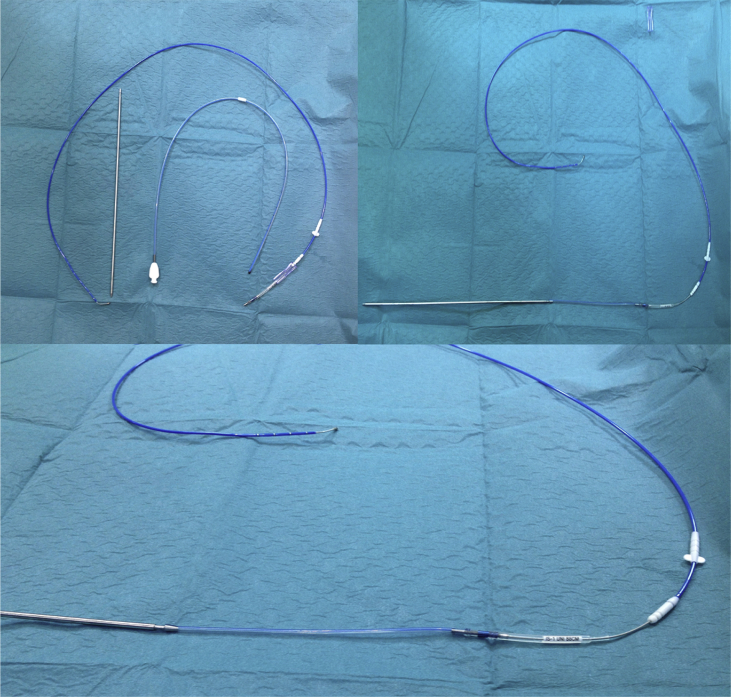
In both cases, the following technique was employed (Fig. 2): A Baird single lumen Groshong central line kit was used. This has a soft, silicone tubing with a closed rounded tip and comes complete with a metal tunnelling device. The closed end of the tubing is excised leaving a double ended silicone tube. One end is pushed onto the rear end of the tunneller and the other end onto the IS-1 connector of the LV lead. The diameter of the Groshong line is a perfect fit for the IS-1 connector. The tunneller is then pushed through the subcutaneous tissue in front of the chest to the contralateral pocket pulling the LV lead IS-1 connector. The diameter of the tunneller is such that the lead pulls freely through the tissue. The lead is then connected to the new CRT-D and the wounds closed with standard technique. A small loop of lead is left in the small pocket side of the venous puncture and the LV lead then secured to the pectoral muscle. Finally, the old device was explanted and the lead parameters checked on the existing leads.
Fig. 2.
Groshong Central line kit used for the procedure.
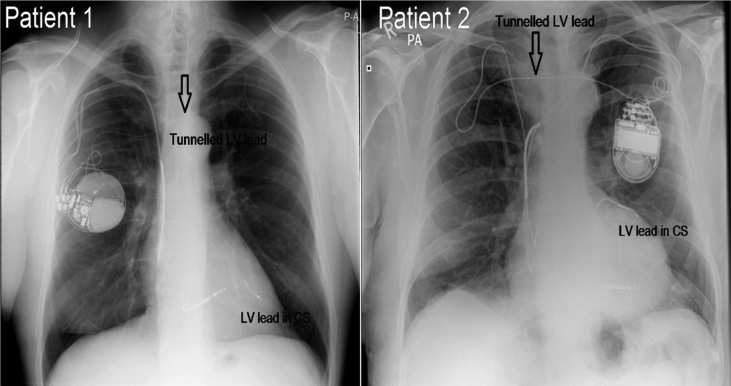
Post-procedure chest X-ray (CXR) confirmed good lead position and no pneumothorax (Fig. 3). At 6 months and 1 year follow-up, there was a significant clinical improvement of heart failure, no procedure related complications and functioning LV lead with stable parameters (Patient 1 LV lead parameters at 6 months: R wave 9.9 mV, Impedance 492 Ω and threshold 0.9 V @ 0.4 ms; and R wave 11.1 mV, Impedance 556 Ω and threshold 0.8 V @ 0.4 ms at 1 year), (Patient 2 LV lead parameters: R wave 11.9 mV, Impedance 627 Ω and threshold 0.5 V @ 0.4 ms at 6 months and R wave of 10.5 mV, Impedance 532 Ω and threshold 0.75 V @ 0.4 ms at 1 year).
Fig. 3.
CXR post-implantation in patient 1 and patient 2.
Discussion
The number of complex device (ICD and CRT-P/D) implantations has significantly increased in the last decade [2], [3]. Not only has the number of new device implantations gone up, also has the number of upgrades either from a pacemaker (PPM) to ICD/CRT or from ICD to CRT-P/CRT-D. Data from the REPLACE registry shows that patients undergoing replacement of a chronically implanted pacemaker or ICD are generally at increased risk for procedure-related complications if a transvenous lead is added to replace a defect electrode or for device upgrade [4]. Of relevance to our patients is the occlusion of the ipsilateral subclavian vein which is not an uncommon finding and previous authors have reported up to 25% of patients needing lead revision or device upgrade [5], [6].
Many alternative routes have been proposed for such a situation: (1) puncture of the ipsilateral subclavian or brachiocepahlic vein proximal (or medial) to the site of obstruction, with or without a supraclavicular approach with subcutaneous tunnelling [7]; (2) Ovadia et al. [8] describe a percutaneous brachiocephalic (innominate) or deep subclavian venous access by a supraclavicular approach using an 18-gauge Deseret angiocath, a Terumo Glidewire, and dilation to permit one or two 9–11 Fr sheaths, followed by tunnelling of electrodes (pre or retroclavicularly) to a pre or retropectoral pocket; (3) Aleksic et al. [9] describe the ‘notch technique’ to puncture of the contralateral innominate vein (with subcutaneous tunnelling) – an 18F needle was introduced 1 cm above the sternoclavicular joint palpable as a notch and angled at 45° to puncture the innominate vein at 3–4 cm depth. A 8.5F percutaneous sheath introducer set (Arrow International Inc) was used and electrode placed with over the wire technique and lead brought back to the original pocket; (4) puncture of the ipsilateral jugular vein from the neck (with subcutaneous tunnelling of the lead) is described by Bosa-Odeja et al. [10] Seldinger technique was used to puncture the jugular vein and a 0.38 inch Teflon guidewire was advanced through the needle to the right ventricle and was replaced with an 8F sheath to continue the procedure in the standard fashion. The lead and the protective sheath were secured to the muscle layers in the clavicular portion of sternocleidomastoid muscle; (5) extraction of the defect lead with ipsilateral implantation of a new transvenous electrode [11]; (6) transatrial or epicardial lead placement [12], [13]; (7) contralateral implantation of the complete system with explantation or abandoning of the old leads [14]; (8) Fox et al. [16] describe the procedure using a pair of long forceps and blunt dissection to create a subcutaneous tract from the right to the left. The proximal end of the LV lead was grasped with the forceps and tunnelled above the sternum to rejoin the pocket; and (9) Another technique not widely practised currently is the placement of transvenous electrode from the contralateral side with sub-cutaneous, pre-sternal lead tunnelling. Luthje et al. [15] described using regular chest tube and a trochar for this technique, and passing the lead in the chest tube to the other end. This procedure is more invasive and involves both the cardiologist and the cardio-thoracic surgeon and is not currently favoured.
Our technique is different and much simpler than the ones described requiring a single operator and a widely available kit. However, more data is needed to assess long term feasibility, safety and reliability before it can be recommended on a universal basis. Because no general recommendations exist currently, the individual choice between the different approaches largely depend on individual operator experience, the indication for lead implantation, patient characteristics, and the resulting risk–benefit ratio. Our technique adds another potential option to patients with limited venous access.
Conflict of interest
All authors have been recipients of travel grants and or bursaries to attend various courses and conferences. There is no specific disclosure relevant to this manuscript.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Dickstein K., Vardas P., Auricchio A., Daubert J.P., Linde C., McMurray J. Focussed update of ESC guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC guidelines for cardiac and resynchronisation therapy developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Eur Heart J. 2010;31:2677–2687. doi: 10.1093/eurheartj/ehq337. [DOI] [PubMed] [Google Scholar]
- 2.Voigt A., Shalaby A., Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol. 2010;33:414–419. doi: 10.1111/j.1540-8159.2009.02569.x. [DOI] [PubMed] [Google Scholar]
- 3.HR UK . 2012. Heart rhythm devices: UK National Clinical Audit 2009. [Ref Type: Internet Communication] [Google Scholar]
- 4.Poole J.E., Mela T., Chung T., Uslan D., Borge R., Gottipaty V. Complication rates associated with pacemaker or implantable cardioverter defibrillator generator replacements and upgrade procedures: results from REPLACE registry. Circulation. 2010;122:1553–1561. doi: 10.1161/CIRCULATIONAHA.110.976076. [DOI] [PubMed] [Google Scholar]
- 5.Haghjoo M., Nikoo M., Fazelifar A., Alizadeh A., Emkanjoo Z., Sadr-Ameli M. Predictors of venous obstruction following pacemaker or implantable cardioverter defibrillator implantation: a contrast venographic study on 100 patients admitted for generator change, lead revision, or device upgrade. Europace. 2007;9:328–332. doi: 10.1093/europace/eum019. [DOI] [PubMed] [Google Scholar]
- 6.Rozmus G., Daubert J.P., Huang D.T., Rosero S., Hall B., Francis C. Venous thrombosis and stenosis after implantation of pacemakers and defibrillators. J Interv Cardiac Electrophysiol. 2005;13:9–19. doi: 10.1007/s10840-005-1140-1. [DOI] [PubMed] [Google Scholar]
- 7.Antonelli D., Freeddberg N., Turgeman Y. Supraclavicular vein approach to overcoming ipsilateral chronic subclavian vein obstruction during pacemaker-ICD lead revision or upgrading. Europace. 2010;12:1596–1599. doi: 10.1093/europace/euq314. [DOI] [PubMed] [Google Scholar]
- 8.Ovadia M., Cooper R.S., Parnell V.A., Dicapua D., Vatsia S.K., Vlay S.C. Transvenous pacemaker insertion ispilateral to chronic subclavian vein obstruction: an operative technique for children and adults. Pacing Clin Electrophysiol. 2000;23:1585–1593. doi: 10.1046/j.1460-9592.2000.01585.x. [DOI] [PubMed] [Google Scholar]
- 9.Aleksic I., Kottenberg-Assenmacher E., Kienbaum P., Szabo A., Sommer S., Wienke H. The innominate vein as alternative venous access for complicated implantable cardioverter defibrillator revisions. Pacing Clin Electrophysiol. 2007;30:957–960. doi: 10.1111/j.1540-8159.2007.00792.x. [DOI] [PubMed] [Google Scholar]
- 10.Bosa-Odeja F., Bethencourt-Munoz M., Vergara-Torres M., Lara-Paoron A., Rodriguez-Gonzales A., Marrero-Rodriguez F. Upgrade of a pacemaker defibrillator to a biventricular device: the internal jugular approach in a case of bilateral subclavian veins occlusion. J Interv Cardiac Electrophysiol. 2007;19:209–211. doi: 10.1007/s10840-007-9149-2. [DOI] [PubMed] [Google Scholar]
- 11.Bracke F., van Gelder L., Sreeram N., Meijer A. Exchange of pacing or defibrillator leads following laser sheath extraction of non-functional leads in patients with ipsilateral obstructed venous access. Heart. 2000;83:e12. doi: 10.1136/heart.83.6.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaroszewski D., Altemose G., Scott L., Srivasthan K., DeValeria P., Lackey J. Non traditional surgical approaches for implantation of pacemaker and cardioverter defibrillator systems in patients with limited venous access. Ann Thorasic Surg. 2009;88:112–116. doi: 10.1016/j.athoracsur.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Molina J. Surgical options for endocardial lead placement when upper veins are obstructed or nonusable. J Interv Cardiac Electrophysiol. 2004;11:149–154. doi: 10.1023/B:JICE.0000042354.87946.39. [DOI] [PubMed] [Google Scholar]
- 14.Ellenbogen K.A., Kay G., Lau C., Wilkoff B.L. Saunders/Elsevier; Philadelphia: 2007. Permanent pacemaker and implantable cardioverter defibrillator implantation. [Google Scholar]
- 15.Luthje L., Zabel M., Seegers D., Vollmann D. Acute and long term feasibility of contralateral transvenous lead replacement with sub-cutaneous, pre-sternal tunnelling in patients with chronically implanted rhythm devices. Europace. 2011;13:1008. doi: 10.1093/europace/eur072. [DOI] [PubMed] [Google Scholar]
- 16.Fox D.J., Petkar S., Davidson N.C., Fitzpatrick A.P. Upgrading patients with chronic defibrillator leads to a biventricular system and reducing patient risk: contralateral LV lead placement. PACE. 2006;29:1025–1027. doi: 10.1111/j.1540-8159.2006.00482.x. [DOI] [PubMed] [Google Scholar]