Figure 4.
Induction of Tumor Antigen-Specific CTLs via Re-iNKT Cell-DC Interaction
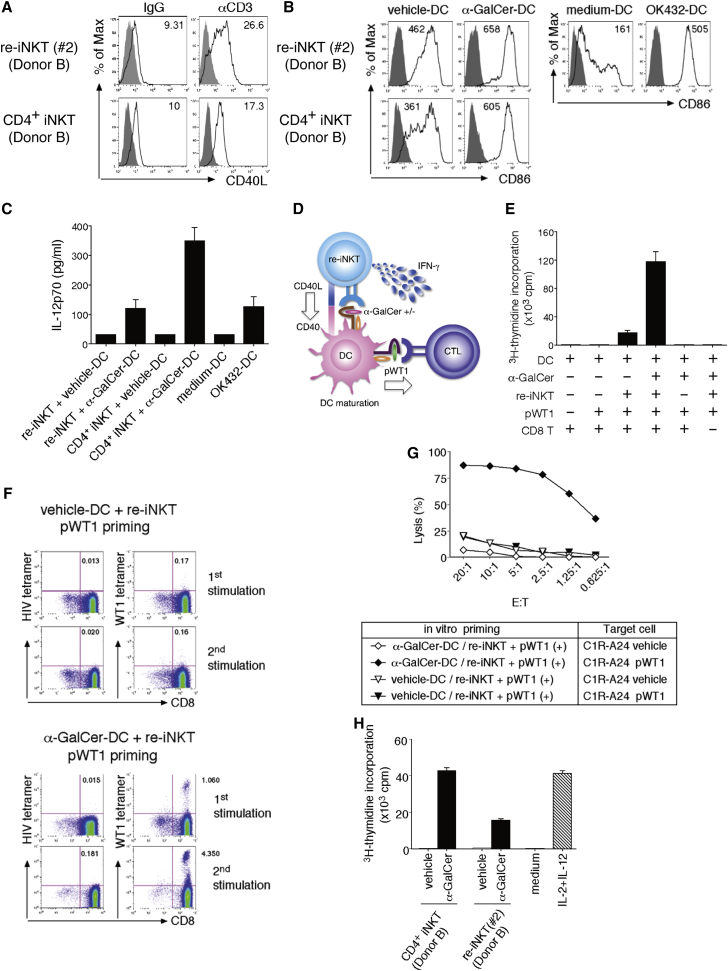
(A) Re-iNKT cells or parental iNKT cells were stimulated with plate-bound anti-CD3 mAb, after which CD40L expression was evaluated. The mean fluorescence intensity (MFI) of CD40L is shown.
(B) Vehicle- or α-GalCer DCs were cultured with re-iNKT cells at a DC/re-iNKT cell ratio of 10:1, after which CD86 expression on DCs was evaluated. The MFI of CD86 is shown. Medium DCs and OK432 DCs served as references.
(C) IL-12p70 production by DCs stimulated using the indicated conditions.
(D) Schematic representation of the WT1235–243-specific CTL priming assay.
(E and F) Vehicle- or α-GalCer DCs were cultured with or without re-iNKT cells (clone 2). Differentially conditioned DCs were irradiated and cultured with autologous CD8+ T cells ± WT1235–243 peptide. (E) Proliferative responses were measured as [3H]thymidine incorporation. (F) Increased frequencies of WT1235–243-specific CTLs after repeated stimulation were determined using WT1 tetramer. HIV tetramer served as a negative control.
(G) Cytotoxic activities of pWT1-primed CD8+ T cells toward C1R-A∗24:02 cells ± WT1 peptide were determined using 51Cr-release assays at the indicated E/T ratios.
(H) Proliferative responses of NK cells (1.0 × 105) cultured for 48 hr in the presence of 25% cell-free supernatants that were from iNKT-DC co-culture. Medium control and IL-2 (300 IU/ml) plus IL-12 (20 ng/ml) control served as references.
In (A), (B), (F), and (G), one representative result from at least two independent experiments is shown. In (C), (E), and (H) data were run in triplicate, and experiments were repeated at least twice; the results of one representative experiment are shown. Error bars depict mean ± SD.