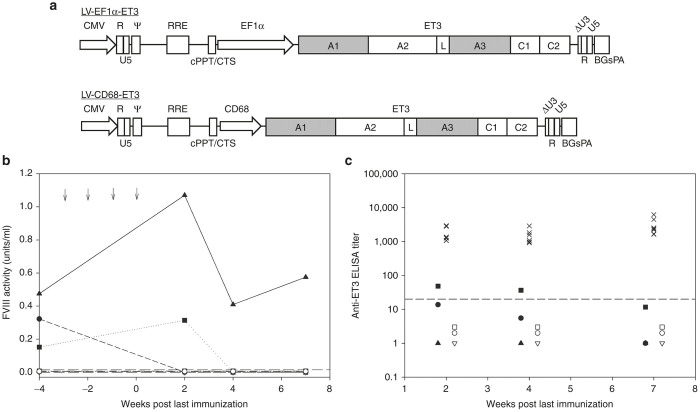
Figure 1.
Immunological challenge following LV-FVIII gene therapy. (a) Schematic of FVIII-LV design. (b) Baseline FVIII activity was determined in LV-CD68-ET3 (closed symbols, each symbol represents an individual mouse) and LV-EF1α-ET3 (open symbols, each symbol represents an individual mouse) hematopoietic stem cell-transplanted (HSCT) mice prior to immunological challenges and subsequently measured after four weekly immunizations (at weeks −3, −2, −1, and 0 indicated by arrows) of 1 µg recombinant ET3. Dashed line indicates limit of detection. (c) Anti-FVIII IgG titers were measured by ELISA and are shown for LV-CD68-ET3 (closed symbols, each symbol represents an individual mouse) and LV-EF1α-ET3 (open symbols, each symbol represents an individual mouse) HSCT-treated mice following the final immunization as well as the immunized controls (×). All mice received four weekly injections of 1 µg of recombinant ET3 posttransplant. The data represent 2, 4, and 7 weeks post final immunization (n = 3 for each experimental cohort, n = 8 for the control cohort). Dashed line indicates limit of detection. A1, A2, A3, C1, and C2, functional domains of a FVIII molecule; BGsPA, bovine growth hormone synthetic polyadenylation tail; CD68, myeloid restricted promoter sequence from the human CD68 locus; CMV, cytomegalovirus promoter; cPPT/CTS, central polypurine tract/central termination sequence; EF1α, elongation factor 1α promoter; L, linker sequence; LV, lentiviral; R, U5, and U3, subcomponents of the 5′ and 3′ long terminal repeats; RRE, Rev protein response element; Ψ (psi), target RNA-site signaling viral packaging.