Fig. 2.
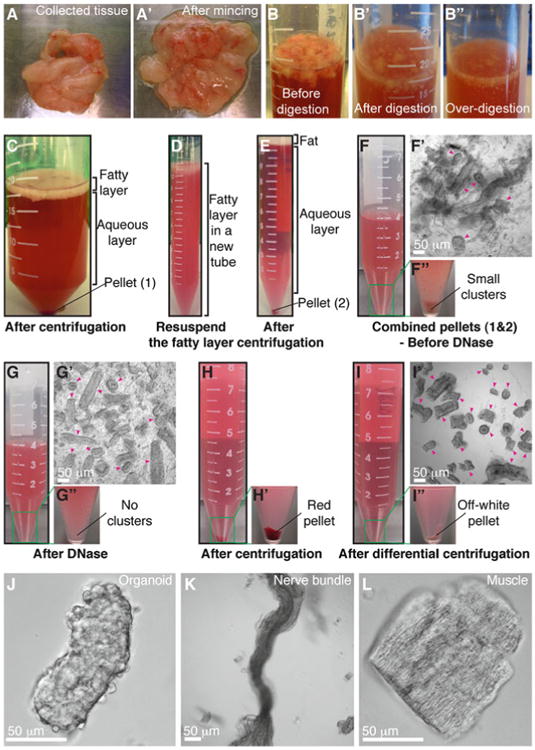
Mammary organoid isolation. (a–a′) Collected mammary glands are pooled in a Petri dish (a) and minced until the tissue relaxes, typically 25–50 cuts (a′). (b–b″) Incubation in collagenase solution breaks up the fat pad (b) into smaller pieces that are relatively dispersed (b′). Too long of a digestion (b″) will cause organoids to be too small and not grow well. (c) Following incubation in collagenase solution, centrifugation separates the suspension into three layers, with a top opaque layer of fat and a pellet (#1) of epithelium and stroma. (d) The fatty layer is transferred to a new tube and resuspended in 10 mL DMEM/F12. (e) Centrifugation of the dispersed fatty layer recovers additional epithelium in the pellet (#2). (f–f″) The combined pellets from (c) and (e) are resuspended in 4 mL DMEM/F12 with DNase (f). Before DNase treatment, organoids (pink arrowheads) are loosely attached to each other and to stromal cells (f′), forming visible clusters in the tube (f″). (g–g″) DNase treatment causes organoids (pink arrowheads) to detach from one another (g′) and the clusters to disappear (g″). (h–h′) Centrifugation of the suspension in (g) results in a compact red pellet (h′). (i–i″) Differential centrifugation removes single cells from the suspension (i′) and results in an off-white pellet of purified epithelial organoids (i″). Organoids (pink arrowheads) may appear rounded and small or more elongated and even branched (i′). Larger organoids typically survive and branch more efficiently in our assays. (j) Close-up view of an organoid. (k–l) Non-epithelial tissues can be observed in the final suspension, including nerve bundles (k) and muscle (l) (Color figure online)
