Figure 1.
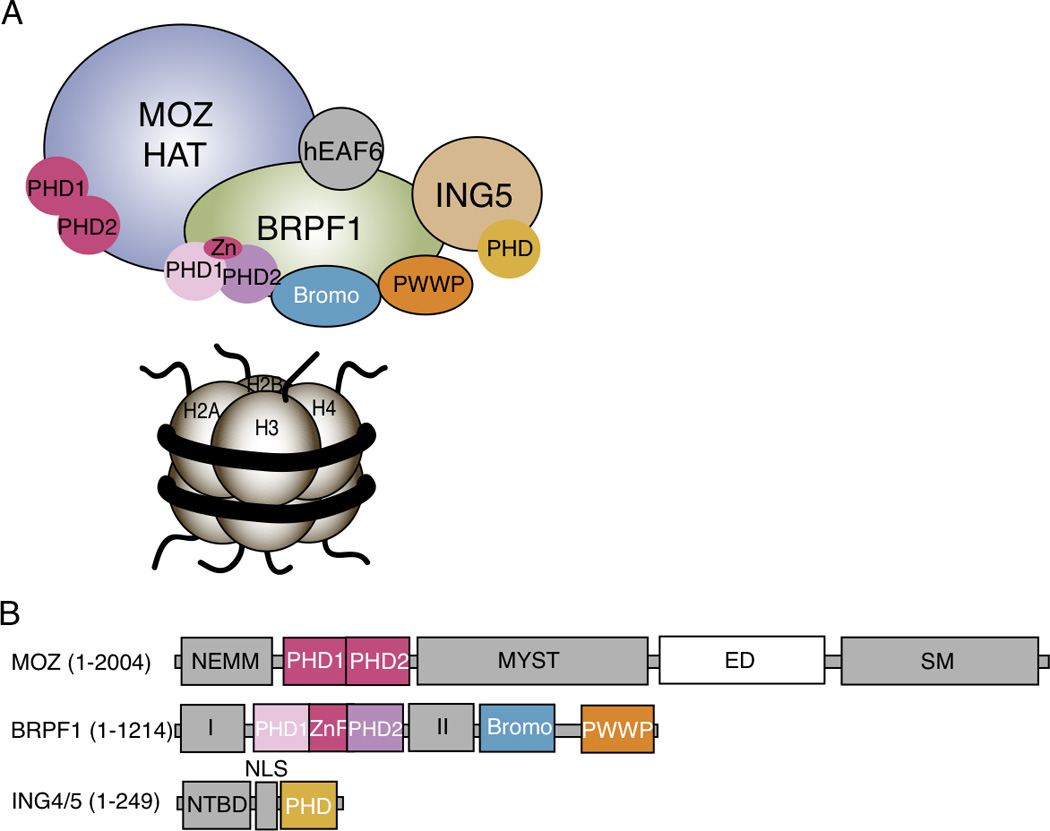
The MOZ HAT complex and subunit architecture. A. The MOZ HAT complex is a hetero-tetramer composed of the monocytic leukemic zinc-finger (MOZ) catalytic subunit, the bromodomain-PHD finger protein 1 (BRPF1), inhibitor of growth 5 (ING5) and hEaf6 subunits. B. The MOZ catalytic domain is a large, multi-domain protein (~250 kDa) possessing HAT activity via its MYST domain, in addition to multiple protein interaction domains including a N-terminal part of Enok, MOZ, or MORF (NEMM) domain, a tandem PHD finger region, a Glu/Asp rich region (ED) and a Ser/Met rich region (SM). The BRPF1 subunit contains multiple chromatin reader domains including a double PHD and zinc finger (ZnF) assembly (PZP), a bromodomain and a chromo/Tudor-related PWWP domain. BRPF1 also includes two protein interaction domains (I and II), which contact MOZ and ING5/Eaf6, respectively. The ING5 subunit contains an N-terminal binding domain (NTBD) that contacts BRPF1, a nuclear localization signal (NLS) and a C-terminal PHD finger.
