Abstract
The junctional adhesion molecule (JAMs) family belongs to the immunoglobulin subfamily involved in the formation of tight junctions (TJ) in both endothelial and epithelial cells. Aberrant expression of JAM-2 is associated with cancer progression but little work has been carried out in discovering how this affects changes in cell behaviour. The present study aimed to examine the expression of JAM-2 in human colon cancer specimens and cell lines and its role in the development of colon cancer. JAM-2 expression in human colon cancer specimens (normal, n=75; cancer, n=94) and cell lines was analysed using quantitative real-time PCR and conventional RT-PCR. Colon cancer cells were stably transfected with a mammalian expression vector to overexpress JAM-2-Flag. The effect on growth, adhesion and migration following overexpression of JAM-2 was then investigated using in vitro models. TJ function was assessed using a trans-epithelial resistance assay (TER, with an EVOM voltammeter). JAM-2 was lowly expressed in colon cancer cells such as RKO, HT115. JAM-2 overexpression in RKO cells (RKO-JAM-2) and HT115 cells (HT115-JAM-2) showed retarded adhesion (P<0.05). An in vivo tumour model showed that RKO-JAM-2 had significantly reduced growth (P<0.05), invasion (P<0.05) and migration (P<0.05) as well as in HT115-JAM-2, except on proliferation and migration. Expression of JAM-2 resulted in a significant increase in TER and decrease in permeability of polarized monolayers (P<0.05). Further analysis of JAM-2 transcript levels against clinical aspects demonstrated that the decreasing JAM-2 expression correlated to disease progression, metastasis and poor survival. Taken together, JAM-2 may function as a putative tumour suppressor in the progression and metastasis of colorectal cancer.
Keywords: metastasis suppressor gene, JAM-2, tight junction, colorectal cancer, migration, invasion, MMP, trans-epithelial resistance
Introduction
Colorectal cancer (CRC), one of the most common malignancies, is the fourth leading cause of cancer-related death globally (1). In China, there is an increasing trend in both morbidity and mortality rates of CRC (2). Although early diagnosis and enhanced surgical resections have increased survival rates in patients who are diagnosed with CRC, almost 50% of patients with CRC will die due to complications associated with metastasis (3). Understanding the mechanisms of metastatic disease has important implications in formulating the treatment and metastatic prevention strategies.
The junctional adhesion molecule (JAMs) family belongs to an immunoglobulin subfamily involved in the formation of tight junctions (TJ) in both endothelial and epithelial cells and are characterized by two immunoglobulin-like domains (V-C2 type Ig domains) (4,5). There are four main members of the JAM protein family, which have been named; JAM-A, JAM-B, JAM-C and JAM-L (6). Junctional adhesion molecule-B (JAMB), also known as JAM-2, is specifically localized to cell-cell contacts and enriched at TJ with homotypic interactions. It is mainly expressed in the heart, endothelium, trophoblasts of the placenta, high endothelial venules and in the endothelium of arterioles (7,8). JAM-2 has multiple functions involved in regulation of endothelial and epithelial paracellular permeability, leukocyte recruitment during inflammation, angiogenesis, cell proliferation and migration (9).
The hallmarks of a metastatic cancer cells are their ability to seperate from the original tumour and enter the circulation via intravasation, leading to the development of tumour metastasis, anaplasia of the primary tumour or lymphovascular invasion (10). The metastatic and invasive cascade consists of many complex cellular interactions and pathways (11). Approximately 90% of all cancer patients experience tumour metastasis (12). Due to the role JAM-2 plays in integrity of junctions between cells, it has become an interesting target for further research. It has been reported that JAM-2 interacts with JAM-3 in leukocytes involved in leukocyte trafficking (13–15). It has been shown that JAM-2 can regulate invasion and metastasis of melanoma with the expression of JAM-3 (16). Also, JAM-2 increases JAM-3-dependent gastric adenocarcinoma tumor metastasis (17). Moreover, JAM-2 interferes with the signaling pathway of angiogenic VEGF/VEGFR2 (18). A previous study suggested that JAM-2 plays an important role in regulating tumor growth and angiogenesis (19). An interesting finding was that JAM-2 has low expression in colorectal cancer due to hypermethylation of its promotor (20,21). In this study we used JAM-2 overexpression in colon cancer cells to study its role in the development of colon tumour progression.
Materials and methods
Human colorectal specimens
A total of 169 patient tissues (94 were colorectal cancer tissues; 75 were normal background tissues) were collected immediately after surgery and snap-frozen in liquid nitrogen until further use. Background normal mammary tissues were gained from the same patients. The size of tumour tissues and normal tissues was confirmed by a pathologist and the background tissues were free of tumour deposits. All protocols were approved by the local ethics committee. Full details of patient clinical data are shown in Table I.
Table I.
Correlation of mRNA of JAM-2 and clinical parameters.
| Category | No. | Median | IQR | P-value |
|---|---|---|---|---|
| T/N | ||||
| Normal | 75 | 6.4 | <0.000001–616 | |
| Tumour | 94 | <0.000001 | <0.000001 | 0.042 |
| Paired T-N | ||||
| Paired normal | 68 | 6.4 | <0.000001–525 | |
| Paired tumour | 68 | <0.000001 | <0.000001–0.01 | 0.03 |
| Location | ||||
| Left colon | 22 | <0.000001 | <0.000001–0.01 | |
| Right colon | 28 | <0.000001 | <0.000001 | |
| Trans-colon | 2 | 0.00175 | N/A | |
| Rectum | 22 | <0.000001 | <0.000001 | |
| Dukes' stage | ||||
| A | 7 | <0.000001 | <0.000001–0.0008 | |
| B | 33 | 3.19 | <0.000001–0.01 | 0.2 |
| C | 32 | 12.8 | <0.000001 | 0.34 |
| BC | 65 | 7.93 | <0.000001–0.01 | 0.17 |
| Tumour stage | ||||
| T1 | 2 | <0.000001 | N/A | |
| T2 | 10 | <0.000001 | <0.000001–0.00745 | 0.19 |
| T3 | 40 | <0.000001 | <0.000001–0.03 | 0.25 |
| T4 | 18 | <0.000001 | <0.000001 | 0.36 |
| T23 | 50 | <0.000001 | <0.000001–0.02 | 0.09 |
| T34 | 58 | <0.000001 | <0.000001–0.01 | 0.27 |
| Lymph node involvement stage | ||||
| N0 | 39 | <0.000001 | <0.000001–0.01 | |
| N1 | 16 | <0.000001 | <0.000001–0.6 | 0.33 |
| N2 | 15 | <0.000001 | <0.000001–0.028 | 0.19 |
| TNM stage | ||||
| I | 9 | <0.000001 | <0.000001–0.01 | |
| II | 30 | <0.000001 | <0.000001–0.01 | 0.54 |
| III&IV | 32 | <0.000001 | <0.000001 | 0.32 |
| Clinical outcome | ||||
| No invasion | 50 | <0.000001 | <0.000001–0.01 | |
| Invasion | 26 | <0.000001 | <0.000001 | |
| Disease free | 35 | 0.001 | <0.000001–0.012 | |
| Incidence | 23 | <0.000001 | <0.000001–0.32 | |
| No metastasis | 50 | <0.000001 | <0.000001–0.008 | |
| Alive | 36 | <0.000001 | <0.000001–0.009 | |
| Died | 22 | <0.000001 | <0.000001–0.17 | |
Cell culture
RKO and HT115 human cancer cell lines were acquired from the European Collection of Animal Cell Cultures (ECACC; Salisbury, UK). These two wild-type cells were routinely cultured in DMEM/F12 HAM (Dulbecco's modified Eagle's medium; Sigma-Aldrich) supplemented with 10% fetal calf serum (FCS; PAA Laboratories, Somerset, UK), penicillin and streptomycin (Sigma-Aldrich), in an incubator at 37.0°C, 95% humidity and 5% CO2.
Construction of JAM-2 expression vectors and transfection
The JAM-2-GFP and pCMV-entry plasmids were purchased from OriGene Technoloqies (Rockville, MD, USA). The JAM-2 cDNA sequences which were obtained from the JAM-2-GFP plasmid were individually cloned into a mammalian expression pCMV-entry plasmid vector. Purified JAM-2 transgenes and control plasmid vectors were transfected into RKO and HT115 cells respectively using an Easjet Plus electroporator (EquiBio Ltd., Kent, UK).
RNA isolation and reverse transcription PCR
RNA was isolated from wild-type and selected cells using total-RNA isolation reagent (Sigma-Aldrich, Dorset, UK). Following the manufacturer's protocol, cDNA was generated from the total RNA using GoScript™ Reverse Transcription System kit (Promega, Madison, WI, USA). Subsequently, PCR was conducted using a REDTaq™ ReadyMix PCR reaction mix (primer sequences shown in Table II). Reaction conditions were: 94°C for 5 min (initial denaturation), followed by 35 cycles of 94°C for 30 sec, 55°C for 30 sec and 72°C for 40 sec. This was followed by a final 10-min extension period at 72°C. The products were visualized on 2% agarose gel stained with ethidium bromide. Quantitative analysis of the JAM-2 transcript was carried out using real-time quantitative PCR with on the Amplifluor™ technology. Q-PCR primers (Table II) were designed using Beacon Design software (Premier Biosoft, Palo Alto, CA, USA), and one of the primers carried a Z-sequence. Colorectal cDNA samples were synchronously examined for JAM-2 and GAPDH along with a set of internal control. Real-time PCR was carried out using iCycler iQ™ (Bio-Rad Laboratories, Hemel Hempstead, UK) following the cycling conditions: 94°C for 5 min, 70 cycles of: 94°C for 10 sec, 55°C for 35 sec and 72°C for 20 sec.
Table II.
Primer sequences.
| Molecule | Sense primers (5′-3′) | Antisense primers (5′-3′) |
|---|---|---|
| JAM-2 (Q-PCR) | TGATAGGGGCTGTAAATCT | ACTGAACCTGACCGTACATAATGATGCAAGACAGTTCC |
| GAPDH (Q-PCR) | CTGAGTACGTCGTGGAGTC | ACTGAACCTGACCGTACACAGAGATGATGACCCTTTTG |
| JAM-2 | GAACTGTGGTAGAGCTACGATGTC | TTTCACTCATTGTCGTGGCTTTAG |
| GAPDH | GGCTGCTTTTAACTCTGGTA | GACTGTGGTCATGAGTCCTT |
Western blot analysis
Total protein concentrations were determined with the DC Protein Assay kit (Bio-Rad Laboratories, Hertfordshire, UK) and an ELx800 spectrophotometer (Elx800 ™; Bio-Tek, Swindon, UK). Equal amounts of protein sample were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto PVDF membrane. Following protein transfer, the membrane was blocked with 5% skimmed milk for 1 h. Proteins were then separately probed with the polyclonal goat anti-human JAM-2 antibody (R&D Systems, Inc., Minneapolis, MN, USA), monoclonal mouse anti-human GAPDH antibody (BD Biosciences, San Diego, CA, USA) and corresponding peroxidase-conjugated secondary antibody. Protein bands were visualized and analysed using Luminata Forte (Merck Millipore, Hertfordshire, UK) and an UVITech imager (UVITech, Inc., Cambridge, UK).
In vitro cell function assays
In vitro cell growth assay
Cell suspensions were added into a 96-well plate (3,000 cells/200 μl/well). Cell growth was assessed after a period of incubation (up to 5 days) in quadruplicate (overnight, day 3, day 4 and day 5). Cells were fixed in 4% formalin and stained with 0.5% crystal violet. Crystal violet was extracted with 10% (v/v) acetic acid and the absorbance of the dissolved dye determined using an ELx800 spectrophotometer (ELx800; Bio-Tek) at a wavelength of 540 nm.
In vitro cell migration assay
Cells (700,000/well) were seeded in a 24-well plate and cultured in the incubation overnight, then scratched with a 10-μl pipette tip to create a wound and washed twice with PBS to remove floating cells. The cells were photographed at intervals using an inverted microscope; the size of the wounds were subsequently measured with the ImageJ software.
In vitro cell adhesion assay
Plates (96-well) were pre-coated with Matrigel (BD Matrigel™ Basement Membrane Matrix) (5 μg/100 μl/well) diluted with serum free media and dried. Following rehydration with serum-free media 40,000 cells were added into each well. After incubation for 40 min, the wells were washed with BSS to remove non-adherent cells and the adherent cells were fixed with 4% formalin and stained with 0.5% crystal violet. Crystal violet staining was dissolved with 10% acetic acid and measured using a spectrophotometer (Elx800; Bio-Tek).
In vitro cell invasion assay
Transwell inserts with an 8 μm pore size were coated with 50 μg Matrigel/100 μl (BD Matrigel™ Basement Membrane Matrix) and air-dried. Following rehydration, 30,000 cells/200 μl/well were added with 0.5% FCS and 1 ml with 10% FCS medium in the bottom well. After 48 h, the cells that migrated through the matrix and pores were fixed with 4% formalin, stained in crystal violet and analyzed.
Transepithelial resistance (TER)
TER was measured with an EVOM Volt-Ohm meter (EVOL, World Precision Instruments, Aston, Herts, UK) with STX2 electrode (World Precision Instruments, Inc., Sarasota, FL, USA). Briefly, cells were added into the 0.4-μm pore size insert (Greiner Bio-One Ltd., Stonehouse, UK) and allowed to reach full confluency. Electrodes were placed at the upper and lower chambers, then resistance was measured with the Volt-Ohm meter. Fluorescein isothiocyanate (FITC)-dextran 10 kDa and TRITC dextran 40 kDa were applied to cells apically. Basolateral dextran passage was analyzed with a GloMax®-Multi Microplate Multimode reader (Promega UK Ltd., Southampton, UK).
Gelatin zymography assay
Cells (1×106) were seeded into a tissue culture flask and cultured in serum-free medium. After 6 h, samples were centrifuged (4,000 rmp for 10 min) to remove cell debris. The supernatant was collected and centrifuged for 15 min (14,000 × g) in Amicon Ultra-0.5 ml centrifugal filters (Merck Millipore, East Midlands, UK). Thereafter, the sample was separated via 10% SDS-PAGE containing 0.1% gelatin. Following electrophoresis, gels were washed and incubated overnight at 37°C. After incubation, gels were stained with Coomassie blue and ubsequently scanned on digital scanner images (SNP-id 2.0 machine; Millipore UK Ltd., Watford, UK) and digital data were saved for analysis.
Statistical analysis
Experimental procedures were repeated independently at least 3 times. Statistical analysis was performed using the Minitab statistical software package (version 14). Data with a non-parametric distribution were assessed with the Mann-Whitney U test, whilst a two sample t-test was used for normally distributed data. Differences were considered to be statistically significant at P<0.05.
Results
JAM-2 expression in colorectal cancer cells and tissues
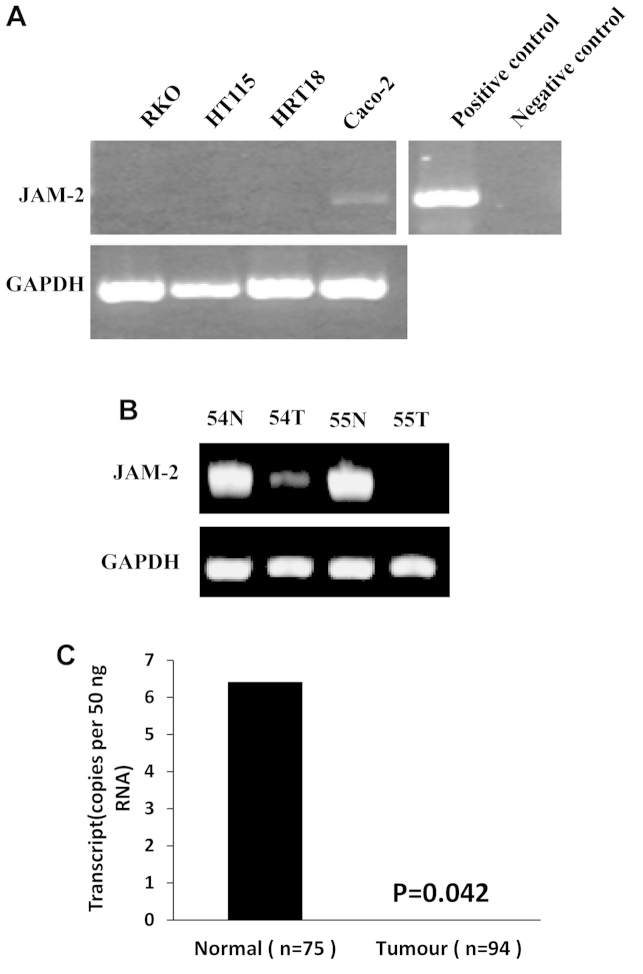
The expression of JAM-2 was examined in colon cancer cell lines and a cohort of colon tumour tissues using reverse transcription-PCR. We found that JAM-2 exhibited very low expression in all four colon cancer cell lines and in colon cancer tissues which were selected randomly (Fig. 1A). JAM-2 transcript levels were quantified in the colon specimens (tumour, n=94; background, n=75) using real-time quantitative PCR (all values displayed as mean JAM-2 transcript copies/μl of cDNA from 50 ng total RNA). In comparison with normal colon tissues, a significantly decreased expression (P=0.042) of JAM-2 was observed in tumour tissues (Fig. 1B).
Figure 1.
Expression of JAM-2 in colon cancer cells and tissues. (A) The mRNA levels of JAM-2 in colon cancer cell lines were barely detectable. (B) JAM-2 mRNA levels were decreased in colon cancer tissues. (C) Expression of JAM-2 in colon cancer tissues. JAM-2 transcript level was decreased significantly in human colon cancer. *P<0.05.
Correlation of JAM-2 expression in colorectal adenocarcinoma and of the clinical characteristics of the disease
JAM-2 transcripts were analysed in colorectal cancer tissues and adjacent normal tissues using Q-RT-PCR. Decreased levels of JAM-2 demonstrated a lower level in tumours than in normal background tissues (P=0.03). The expression level of JAM-2 decreased as TNM stage (P=0.32) and nodal stage (P=0.19) and levels of JAM-2 transcript were lower in node-positive tumours than in node-negative tumours, lower in tumours with distant metastasis than those without metastasis, and much lower in the patients who had died of colon cancer than in those who remained alive and well, but this did not reach statistical significance. Medical notes and histology reports were used to extract clinico-pathological data (Table I).
Expression of JAM-2 in colon cancer cell lines reduces cell growth, adhesion, migration and invasion
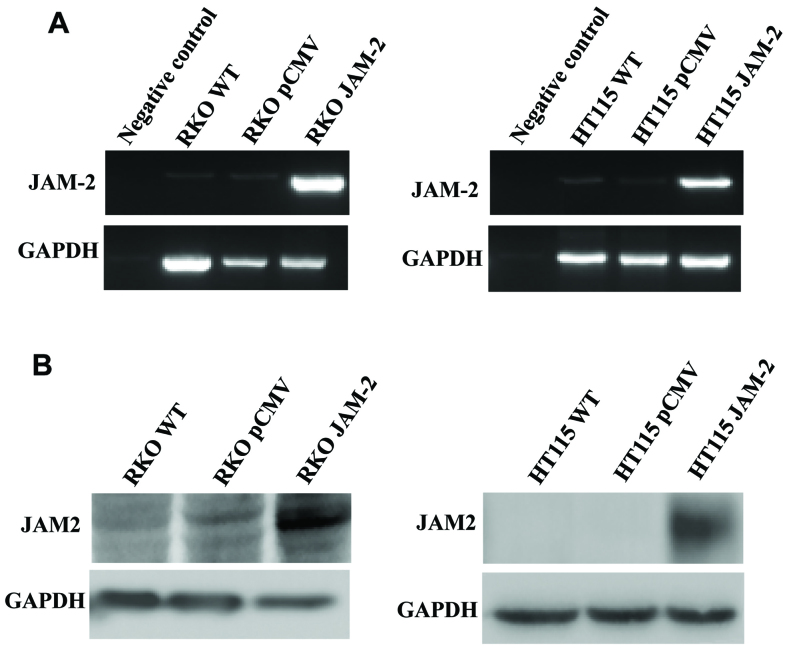
To study the role of JAM-2 in colon cancer metastasis and progression, we over-expressed JAM-2 in RKO and HT115 cells. We confirmed that JAM-2 plasmid had successfully expressed JAM-2 within the RKO and HT115 colon cancer cell lines (Fig. 2). RT-PCR and western blot data demonstrated that JAM-2 was expressed at mRNA and protein level in comparison to the level of expression in the empty plasmid control cells (RKOpCMV-entry and HT115pCMV-entry) and in wild-type cells (RKOWT and HT115WT) (Fig. 2).
Figure 2.
Overexpression of JAM-2 in colon cancer cells. (A) Expression of JAM-2 in RKO and HT115 cells were verified using RT-PCR. (B) Expression of JAM-2 in RKO and HT115 cells was verified using western blotting.
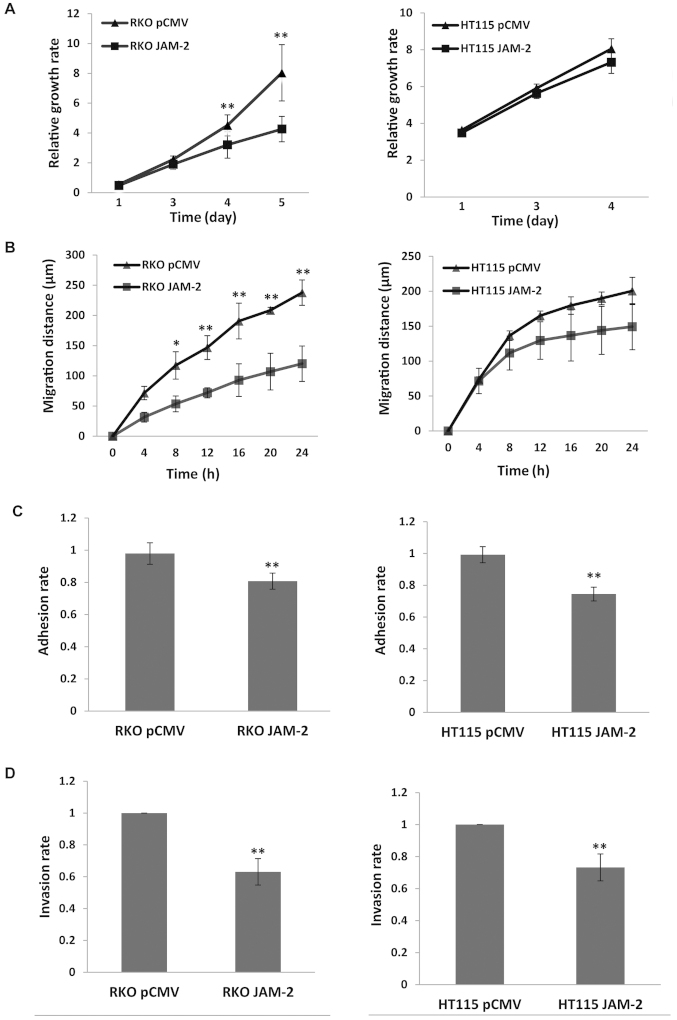
Compared with the pCMV-entry control, JAM-2 expression caused a significant reduction of RKO cell growth (P<0.01) but not in HT115 (P>0.05) cells, however, the growth trend of the two cell lines was similar (Fig. 3A). The migration assay showed that expression of JAM-2 in RKO cells resulted in a strong reduction in the degree of migration (P<0.01 vs. pCMV-entry control) (Fig. 3B) but did not result in a significant change on HT115 cells. We also determined the influence on cell adhesion. Expression of JAM-2 decreased adhesion of the colon cancer cell lines and produced a significant change in both RKO and HT115 cells (P<0.01 vs. pCMV-entry control) (Fig. 3C). Moreover, invasion data suggest that increasing JAM-2 expression depressed colon cancer cells invasion (P<0.01 vs. pCMV-entry control) (Fig. 3D).
Figure 3.
The effect of forced JAM-2 expression on biological functions of colon cancer cells. (A) Forced JAM-2 expression inhibited the in vitro growth of RKO cell and had little effect on HT115. (B) Forced JAM-2 expression in RKO cells had inhibitory effect on RKO cell motility had not significant effect on HT115. (C) Forced JAM-2 expression reduced cell-matrix adhesion of colon cancer cells. (D) Forced JAM-2 expression decreased invasion of colon cancer cells. Shown are representative results of three independent experiments of each function assay. **P<0.01, *P<0.05.
Expression of JAM-2 influences MMP activity in colon cancer cells
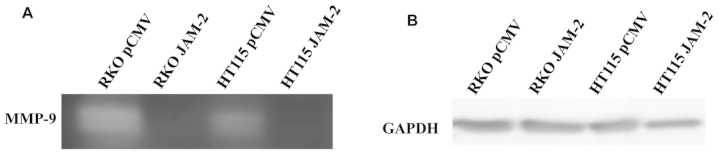
Invasive potential relates to the ability of tumour cells to degrade the extracellular matrix. We used gelatin zymography on supernatants from RKO and HT115 JAM-2 expression cells, which showed a decrease in MMP9 activity compared with pCMV-entry control cells (Fig. 4).
Figure 4.
MMP-9 expression after JAM-2 overexpression. Zymography showed that forced JAM-2 expression led to decreased secretion of MMP-9 by RKO and HT115 cells.
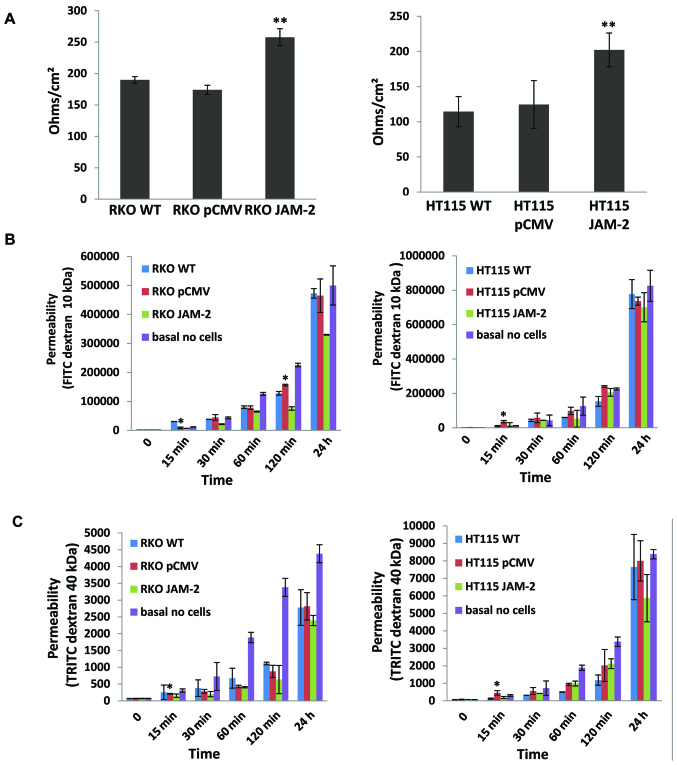
Expression of JAM-2 decreases the trans-epithelial resistance (TER) and the permeability of polarized monolayers
We next examined the effect of JAM-2 overexpression on TJ barrier function. JAM-2 had a significant effect on the TJ function of colon cancer cells. TER in both RKO and HT115 with JAM-2 expression cells were reduced, in comparison to that in empty plasmid control cells (RKOpCMV-entry and HT115pCMV-entry) and in wild-type cells (RKOWT and HT115WT) (P<0.01; Fig. 5A). In order to verify the TER results, we also detected the flux between polarized cells monolayers using paracellular perm-ability. This demonstrated that the higher the TER, the lower the permeability of polarized monolayers was with both FITC dextran 10 kDa and TRITC dextran 40 kDa (Fig. 5B and C).
Figure 5.
Effect of JAM-2 on the behaviour of RKO and HT115 cells. (A) JAM-2 expression increased the TER in both RKO and HT115 monolayer in comparison to that in empty plasmid control cells. (B) JAM-2 inhibits FITC flux of RKO and HT115 monolayer. (C) JAM-2 expression decreased permeability of polarized monolayers of RKO and HT115 cells in TRITC.
Discussion
In the present study, we demonstrated that JAM-2 has low expression in colon cancer which is consistent with previous studies, where it was shown that JAM-2 is downregulated due to the JAM-2 gene having a hyper-methylated promoter at the CpG islands (20,22). We have also shown that JAM-2 expression exerts a significant effect on tumour metastasis and invasion. JAM-2 expression decreases the invasive properties of RKO and HT115 colon cancer in vitro, leading to reduced MMP9 activity.
Regarding to the role of JAM integrity at the junctions between cells, some studies have focused on the expression analysis of the JAM-2 gene in different cancers. Recently, a report showed that the mRNA level of JAM-2 was deregulated in gastric cancer (17). Another study found that JAM-2 occurred in primary cancer compared to chronic gastritis and metastatic cancer (23). Taken together, JAM-2 expression in glioma suggests that JAM-2 may play a role in cancer metastasis by decreasing the expression of actin filament-associated protein through interacting with JAM-3. Arcangeli et al (16) have shown that JAM-2 expression in endothelial cells contributed to murine B16 melanoma cell metastasis through interacting with JAM-C on tumour cells. The present study also detected the expression of JAM-2 in relation to colorectal cancer patient clinical data in a cohort of human colorectal cancer specimens through quantitative PCR. Reduced transcript expression of JAM-2 was observed in the colon cancer tissue sections in comparison to normal background mammary tissues (P=0.042). This indicated that a loss of JAM-2 may occur as cells and normal tissues progress to a cancerous state.
Following overexpression of JAM-2 in colon cancer cells, analysis of functional studies revealed a statistically significant reduction in JAM-2 expression in RKO cells as compared to controls in growth, migration, adhesion and invasion. This occurred in HT115 cells, although the effect on proliferation and migration was not as substantial. Cell-matrix adhesion plays a key step in cancer metastasis and is essential for invasion through matrix so as to progress the metastatic process.
Numerous studies suggest matrix metalloproteases (MMP), a family of multidomain, zinc-containing neutral endopeptidases, to contribute to form a microenvironment that promotes tumour metastasis during early stages of tumourigenesis (24,25). Degradation of extracellular matrix components containing laminin, proteoglycans, collagen and other glycoproteins by MMPs facilitates proliferation, migration and metastasis of cancer cells, via blood and lymphatic routes (26). MMP-9-induced release of biological mediators from the extracellular matrix surrounding a cancer may compose a system by which stromal and neoplastic cells communicate. MMP-2 and MMP-9 are important members of MMPs family and their role has been studied in colon cancer (27). MMP-9 has been related to tumour progression in numerous studies (28–32). MMP-9 is regarded as an extreme enzyme for damage of the basement membrane, the first barrier for tumour invasion. Some studies have demonstrated that it is associated with tumour metastasis (33,34). Our study shows that MMP-9 is downregulated in JAM-2 overexpressing cells, which is consistent with the decreased invasive capability of JAM-2 expression in colon cancer cells.
JAM-2 is involved in the formation of TJs, with increased TER and decreased permeability in several cell types. In this study, resistance in both RKO and HT115 monolayer was reduced with JAM-2 overexpression in comparison to the empty plasmid control cells. Overall, JAM-2 decreased the permeability of polarized monolayers.
In conclusion, we characterized a pattern of low expression of JAM-2 in colon cancers which was correlated with metastasis of colorectal cancer. With cells overexpressing JAM-2, we found that JAM-2 can inhibit tumour metastasis by reducing invasion. This study also showed that overexpression of JAM-2 inhibits the invasive potential of cancer cells by regulating the transcription of MMP-9. JAM-2 provides a new research direction for the diagnosis and treatment of relevant diseases.
Acknowledgements
The present study was supported by the Beijing Municipal Science & Technology Commission (no. Z151100001615039). H.S.Z. was a recipient of the Cardiff University China Medical Scholarship. The authors wish to thank Cancer Research Wales and the Welsh Network of Life Science.
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Lei T, Chen WQ, Zhang SW, Lei TH, Ying Q, He ZY, Wang XH. Prevalence trend of colorectal cancer in 10 cities and counties in China from 1988 to 2002. Zhonghua Zhong Liu Za Zhi. 2009;31:428–433. in Chinese. [PubMed] [Google Scholar]
- 3.de Krijger I, Mekenkamp LJ, Punt CJ, Nagtegaal ID. MicroRNAs in colorectal cancer metastasis. J Pathol. 2011;224:438–447. doi: 10.1002/path.2922. [DOI] [PubMed] [Google Scholar]
- 4.Martìn-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aurrand-Lions M, Johnson-Leger C, Wong C, Du Pasquier L, Imhof BA. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood. 2001;98:3699–3707. doi: 10.1182/blood.V98.13.3699. [DOI] [PubMed] [Google Scholar]
- 6.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 7.Palmeri D, van Zante A, Huang CC, Hemmerich S, Rosen SD. Vascular endothelial junction-associated molecule, a novel member of the immunoglobulin superfamily, is localized to intercellular boundaries of endothelial cells. J Biol Chem. 2000;275:19139–19145. doi: 10.1074/jbc.M003189200. [DOI] [PubMed] [Google Scholar]
- 8.Aurrand-Lions M, Duncan L, Ballestrem C, Imhof BA. JAM-2, a novel immunoglobulin superfamily molecule, expressed by endothelial and lymphatic cells. J Biol Chem. 2001;276:2733–2741. doi: 10.1074/jbc.M005458200. [DOI] [PubMed] [Google Scholar]
- 9.Luissint AC, Nusrat A, Parkos CA. JAM-related proteins in mucosal homeostasis and inflammation. Semin Immunopathol. 2014;36:211–226. doi: 10.1007/s00281-014-0421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brambilla D, Fais S. The Janus-faced role of ezrin in ‘linking’ cells to either normal or metastatic phenotype. Int J Cancer. 2009;125:2239–2245. doi: 10.1002/ijc.24734. [DOI] [PubMed] [Google Scholar]
- 11.Steeg PS. Tumor metastasis: Mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 12.Crnic I, Christofori G. Novel technologies and recent advances in metastasis research. Int J Dev Biol. 2004;48:573–581. doi: 10.1387/ijdb.041809ic. [DOI] [PubMed] [Google Scholar]
- 13.Doñate C, Ody C, McKee T, Ruault-Jungblut S, Fischer N, Ropraz P, Imhof BA, Matthes T. Homing of human B cells to lymphoid organs and B-cell lymphoma engraftment are controlled by cell adhesion molecule JAM-C. Cancer Res. 2013;73:640–651. doi: 10.1158/0008-5472.CAN-12-1756. [DOI] [PubMed] [Google Scholar]
- 14.Liang TW, Chiu HH, Gurney A, Sidle A, Tumas DB, Schow P, Foster J, Klassen T, Dennis K, DeMarco RA, et al. Vascular endothelial-junctional adhesion molecule (VE-JAM)/JAM 2 interacts with T, NK, and dendritic cells through JAM 3. J Immunol. 2002;168:1618–1626. doi: 10.4049/jimmunol.168.4.1618. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig RJ, Zollner TM, Santoso S, Hardt K, Gille J, Baatz H, Johann PS, Pfeffer J, Radeke HH, Schön MP, et al. Junctional adhesion molecules (JAM)-B and -C contribute to leukocyte extravasation to the skin and mediate cutaneous inflammation. J Invest Dermatol. 2005;125:969–976. doi: 10.1111/j.0022-202X.2005.23912.x. [DOI] [PubMed] [Google Scholar]
- 16.Arcangeli ML, Frontera V, Bardin F, Thomassin J, Chetaille B, Adams S, Adams RH, Aurrand-Lions M. The Junctional Adhesion Molecule-B regulates JAM-C-dependent melanoma cell metastasis. FEBS Lett. 2012;586:4046–4051. doi: 10.1016/j.febslet.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Hajjari M, Behmanesh M, Sadeghizadeh M, Zeinoddini M. Junctional adhesion molecules 2 and 3 may potentially be involved in progression of gastric adenocarcinoma tumors. Med Oncol. 2013;30:380. doi: 10.1007/s12032-012-0380-z. [DOI] [PubMed] [Google Scholar]
- 18.Meguenani M, Miljkovic-Licina M, Fagiani E, Ropraz P, Hammel P, Aurrand-Lions M, Adams RH, Christofori G, Imhof BA, Garrido-Urbani S. Junctional adhesion molecule B interferes with angiogenic VEGF/VEGFR2 signaling. FASEB J. 2015;29:3411–3425. doi: 10.1096/fj.15-270223. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds LE, Watson AR, Baker M, Jones TA, D'Amico G, Robinson SD, Joffre C, Garrido-Urbani S, Rodriguez-Manzaneque JC, Martino-Echarri E, et al. Tumour angiogenesis is reduced in the Tc1 mouse model of Down's syndrome. Nature. 2010;466:398. doi: 10.1038/nature09281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kok-Sin T, Mokhtar NM, Ali Hassan NZ, Sagap I, Rose IM, Harun R, Jamal R. Identification of diagnostic markers in colorectal cancer via integrative epigenomics and genomics data. Oncol Rep. 2015;34:22–32. doi: 10.3892/or.2015.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bujko M, Kober P, Mikula M, Ligaj M, Ostrowski J, Siedlecki JA. Expression changes of cell-cell adhesion-related genes in colorectal tumors. Oncol Lett. 2015;9:2463–2470. doi: 10.3892/ol.2015.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bujko M, Kober P, Mikula M, Ligaj M, Ostrowski J, Siedlecki JA. Expression changes of cell-cell adhesion-related genes in colorectal tumors. Oncol Lett. 2015;9:2463–2470. doi: 10.3892/ol.2015.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Huang JY, Chen YN, Yuan F, Zhang H, Yan FH, Wang MJ, Wang G, Su M, Lu G, et al. Whole genome and transcriptome sequencing of matched primary and peritoneal metastatic gastric carcinoma. Sci Rep. 2015;5:13750. doi: 10.1038/srep13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overall CM, López-Otín C. Strategies for MMP inhibition in cancer: Innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 25.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: An overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/A:1026028303196. [DOI] [PubMed] [Google Scholar]
- 26.Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A. Patterns of MMP-2 and MMP-9 expression in human cancer cell lines. Oncol Rep. 2009;21:1323–1333. doi: 10.3892/or_00000358. [DOI] [PubMed] [Google Scholar]
- 27.Frewer KA, Sanders AJ, Owen S, Frewer NC, Hargest R, Jiang WG. A role for WISP2 in colorectal cancer cell invasion and motility. Cancer Genomics Proteomics. 2013;10:187–196. [PubMed] [Google Scholar]
- 28.Arnold S, Mira E, Muneer S, Korpanty G, Beck AW, Holloway SE, Mañes S, Brekken RA. Forced expression of MMP9 rescues the loss of angiogenesis and abrogates metastasis of pancreatic tumors triggered by the absence of host SPARC. Exp Biol Med (Maywood) 2008;233:860–873. doi: 10.3181/0801-RM-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, Giavazzi R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: Implications for ascites formation. Cancer Res. 2003;63:5224–5229. [PubMed] [Google Scholar]
- 30.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HJ, Park CI, Park BW, Lee HD, Jung WH. Expression of MT-1 MMP, MMP2, MMP9 and TIMP2 mRNAs in ductal carcinoma in situ and invasive ductal carcinoma of the breast. Yonsei Med J. 2006;47:333–342. doi: 10.3349/ymj.2006.47.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kondratiev S, Gnepp DR, Yakirevich E, Sabo E, Annino DJ, Rebeiz E, Laver NV. Expression and prognostic role of MMP2, MMP9, MMP13, and MMP14 matrix metalloproteinases in sinonasal and oral malignant melanomas. Hum Pathol. 2008;39:337–343. doi: 10.1016/j.humpath.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 34.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]