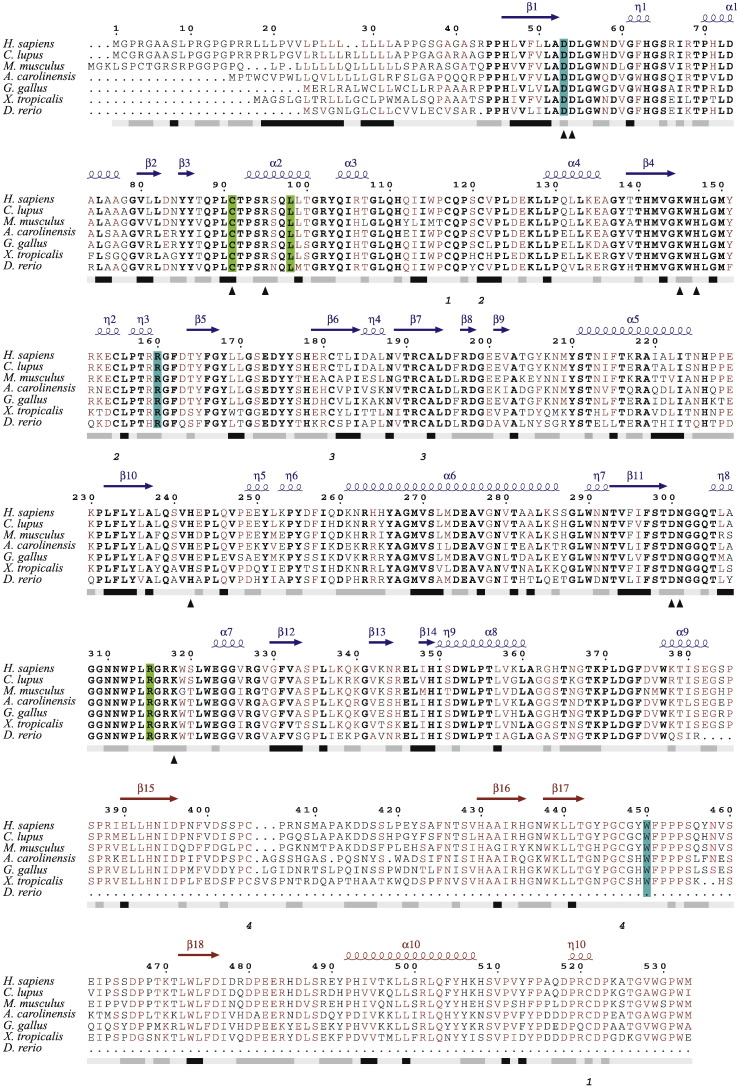
Fig. 2.
Multiple sequence alignment of ARSB. Multiple sequence alignment of ARSB of a few vertebrate species including the human. Bold font represents strictly conserved amino acid residues, sites with sequence identities of 70% or more are in maroon. The bar below the sequence block represents the hydropathy plot. Hydrophobic regions are marked in black, hydrophilic ones in gray & the intermediate regions in faint gray. Cysteine residues forming disulfide bridges are shown below the hydropathy bar. Mutations identified through this study are highlighted in green (novel mutations) or cyan (previously reported). The secondary structure depicted on top of the alignment blocks are inferred from the crystal structure of human ARSB (PDB code: 1FSU). Blue and maroon in the secondary structure cartoon denote the domains 1 and 2, respectively.