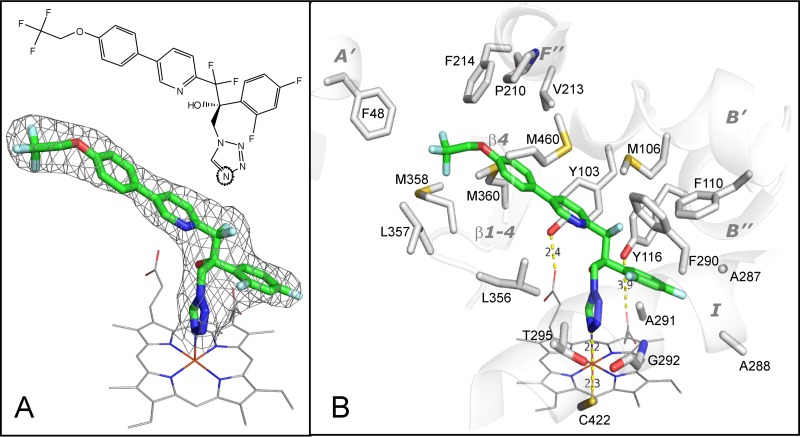
FIG 5.
(A) Structural formula (top) and 2Fo-Fc electron density map (bottom) of VT-1161 coordinated to the T. cruzi CYP51 heme iron. Here and in Fig. 6, VT-1161 is presented as a stick model, and carbon atoms are green. The map is shown as a gray mesh and contoured at 1.3σ. The heme is depicted as a wire model, and carbon atoms are gray. (B) The 19 amino acid residues that surround VT-1161 in the T. cruzi CYP51 active site. The corresponding secondary structural elements of the enzyme are presented as semitransparent ribbons and marked. (The corresponding residues in the aligned sequences of CYP51 from C. albicans and A. fumigatus are listed in Table 2).