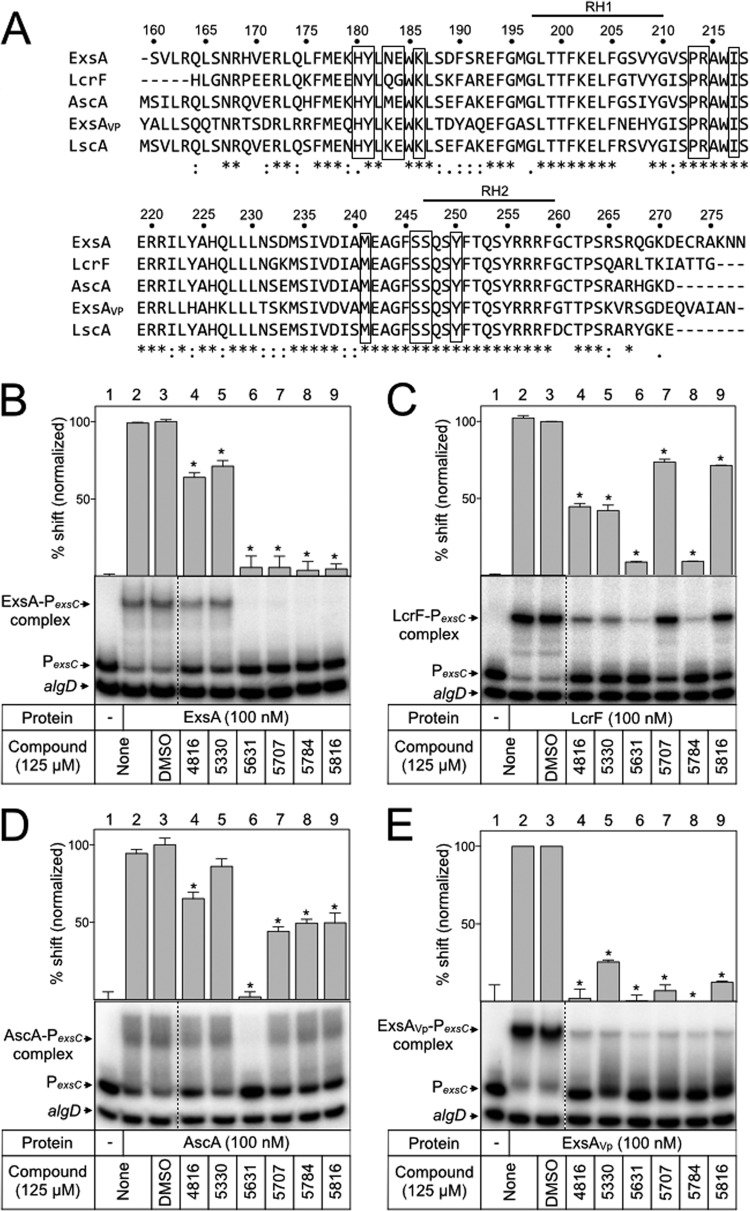
FIG 5.
N-Hydroxybenzimidazoles inhibit the DNA-binding activity of other AraC family proteins. (A) Amino acid sequence alignment of the ExsA DNA-binding domain and homologs in Vibrio parahaemolyticus (ExsAVp), Yersinia pestis (LcrF), Aeromonas hydrophila (AscA), and Photorhabdus luminescens (LscA). Bold lines above the sequences indicate the recognition helices, RH1 and RH2. Boxes outline amino acids that were mutagenized in ExsA. ExsA (B), LcrF (C), AscA (D), and ExsAVp (E) (100 nM) were incubated with DMSO (2.5%) or the indicated N-hydroxybenzimidazole (125 μM) for 5 min prior to addition of radiolabeled specific (PexsC) and nonspecific (algD) probes (0.05 nM each). Binding reactions were allowed to proceed for 15 min at 25°C and then analyzed by native polyacrylamide gel electrophoresis and phosphorimaging. The positions of unshifted probes and shifted protein-DNA complexes are indicated. Quantitation of the percent shift normalized to DMSO-treated protein (100%) is indicated, and statistical differences were determined by comparison to DMSO treatment. *, P < 0.01.