FIG 2.
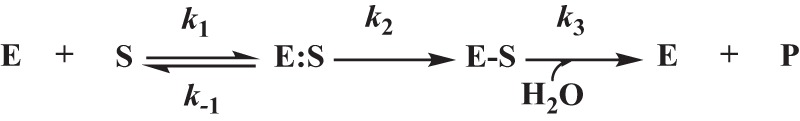
Interactions between the β-lactamase (E) and the β-lactam (S) were initially interpreted according to the scheme shown. Here, the formation of the noncovalent complex, E:S, is represented by the dissociation constant, Ks, which is equivalent to k−1/k1. k2 is the first-order rate constant for the acylation step, or the formation of the E-S complex. k3 is the rate constant for the hydrolysis of the E-S acyl-enzyme and product (P) release. The Michaelis constant, Km, is equivalent to Ks × (k3/k2 + k3).
