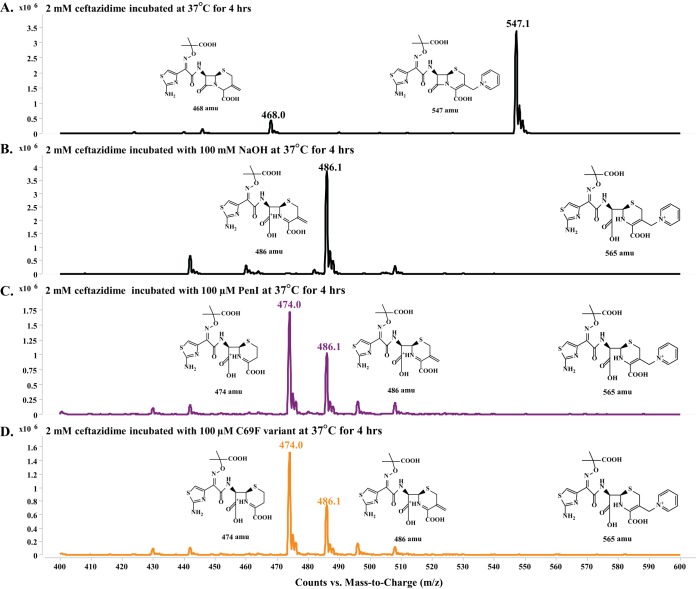
FIG 6.
Small-molecule mass spectrometry revealed the observed products after incubation at 37°C for 4 h, restricting the mass range window to 400 to 600 amu. (A) Ceftazidime (2 mM) alone showed a major peak of the full-size ceftazidime at 547 amu and a very minor peak at 468 amu representative of ceftazidime with the breakdown of the R2 side chain to the exomethylene; increasing the fragmenter and cell accelerator voltage while conducting mass spectrometry can result in artifactual breakdown to the 468-amu ceftazidime. (B) Ceftazidime (2 mM) hydrolyzed by 100 mM NaOH revealed one major peak of 486 amu, corresponding to the hydrolyzed ceftazidime with breakdown of the R2 side chain to the exomethylene. (C) Ceftazidime (2 mM) hydrolyzed by 100 μM PenI identified two peaks of 474 and 486 amu, which correspond to hydrolyzed ceftazidime with complete loss of the R2 side chain and hydrolyzed ceftazidime with breakdown of the R2 side chain to the exomethylene, respectively. (D) Ceftazidime (2 mM) hydrolyzed by 100 μM the C69F variant identified two peaks of 474 and 486 amu, which correspond to hydrolyzed ceftazidime with complete loss of the R2 side chain and hydrolyzed ceftazidime with breakdown of the R2 side chain to the exomethylene, respectively. The full-size hydrolyzed ceftazidime of 565 amu was not observed under any tested condition.