Abstract
Background:
Understanding the related fates of muscle density and bone quality after chronic spinal cord injury (SCI) is an important initial step in determining endocrine-metabolic risk.
Objective:
To examine the associations between muscle density and indices of bone quality at the distal lower extremity of adults with chronic SCI.
Methods:
A secondary data analysis was conducted in 70 adults with chronic SCI (C2-T12; American Spinal Injury Association Impairment Scale [AIS] A-D; ≥2 years post injury). Muscle density and cross-sectional area (CSA) and bone quality indices (trabecular bone mineral density [TbBMD] at the distal tibia [4% site] and cortical thickness [CtTh], cortical area [CtAr], cortical BMD [CtBMD], and polar moment of inertia [PMI] at the tibial shaft [66% site]) were measured using peripheral quantitative computed tomography. Calf lower extremity motor score (cLEMS) was used as a clinical measure of muscle function. Multivariable linear regression analyses were performed to determine the strength of the muscle-bone associations after adjusting for confounding variables (sex, impairment severity [AIS A/B vs AIS C/D], duration of injury, and wheelchair use).
Results:
Muscle density was positively associated with TbBMD (b = 0.85 [0.04, 1.66]), CtTh (b = 0.02 [0.001, 0.034]), and CtBMD (b = 1.70 [0.71, 2.69]) (P < .05). Muscle CSA was most strongly associated with CtAr (b = 2.50 [0.12, 4.88]) and PMI (b = 731.8 [161.7, 1301.9]) (P < .05), whereas cLEMS was most strongly associated with TbBMD (b = 7.69 [4.63, 10.76]) (P < .001).
Conclusion:
Muscle density and function were most strongly associated with TbBMD at the distal tibia in adults with chronic SCI, whereas muscle size was most strongly associated with bone size and geometry at the tibial shaft.
Key words: bone mineral density, bone quality, muscle density, muscle size, osteoporosis, peripheral quantitative computed tomography, spinal cord injury
Spinal cord injury (SCI) is associated with sublesional muscle atrophy,1–3 changes in muscle fiber type,4,5 reductions in hip and knee region bone mineral density (BMD),6–8 and increased central and regional adiposity after injury.9,10 Adverse changes in muscle and bone health in individuals with SCI contribute to an increased risk of osteoporosis,11–13 fragility fractures,14 and endocrine-metabolic disease (eg, diabetes, dyslipidemia, heart disease).15–17 Crosssectional studies have shown a higher prevalence of lower extremity fragility fractures among individuals with SCI ranging from 1% to 34%.18–20 Fragility fractures are associated with negative health and functional outcomes, including an increased risk of morbidity and hospitalization,21,22 mobility limitations,23 and a reduced quality of life.24 Notably, individuals with SCI have a normal life expectancy, yet fracture rates increase annually from 1% per year in the first year to 4.6% per year in individuals greater than 20 years post injury.25,26
Muscle and bone are thought to function as a muscle-bone unit, wherein muscle contractions impose loading forces on bone that produce changes in bone geometry and structure.27,28 A growing body of evidence has shown that individuals with SCI (predominantly those with motor complete injury) exhibit similar patterns of decline in muscle cross-sectional area (CSA) and BMD in the acute and subacute stages following injury.4,11,29 Prospective studies have exhibited a decrease in BMD of 1.1% to 47% per year6,7,30 and up to 73% in the 2 to 7 years following SCI.8,14,31,32 Decreases in muscle CSA have been well-documented following SCI, with greater disuse atrophy observed after complete SCI versus incomplete SCI, presumably due to the absence of voluntary muscle contractions and associated mobility limitations.1,2,16 Muscle quality is also compromised early after SCI, resulting in sublesional accumulation of adipose tissue in the chronic stage of injury3,33,34; the exact time course of this event has been poorly elucidated to date. Adipose tissue deposition within and between skeletal muscle is linked to an increase in noncontractile muscle tissue and a reduction in muscle force-generating capacity on bone.35,36 Skeletal muscle fat infiltration is up to 4 times more likely to occur in individuals with SCI,1,16,37 contributing to metabolic complications (eg, glucose intolerance),16 reduced muscle strength and function,38 and mobility limitations3 – all factors that may be associated with a deterioration in bone quality after SCI.
The association between lean tissue mass and bone size (eg, BMD and bone mineral content) in individuals with SCI has been wellestablished using dual energy x-ray absorptiometry (DXA).9,10,29,34 However, DXA is unable to measure true volumetric BMD (vBMD), bone geometry, and bone structure. Peripheral quantitative computed tomography (pQCT) is an imaging technique that improves our capacity to measure indices of bone quality and muscle density and CSA at fracture-prone sites (eg, tibia).3,39 Recent evidence from cross-sectional pQCT studies has shown that muscle CSA and calf lower extremity motor score (cLEMS) were associated with indices of bone quality at the tibia in individuals with SCI.13,40 However, neither study measured muscle density (a surrogate of fatty infiltration when evaluating the functional muscle-bone unit). Fatty infiltration of muscle is common after SCI1,16,37 and may affect muscle function or the muscle-bone unit, but the association between muscle density and bone quality indices at the tibia in individuals with chronic SCI is unclear. Muscle density measured using pQCT may be an acceptable surrogate of muscle quality when it is difficult to assess muscle strength due to paralysis.3,39 Additionally, investigating which muscle outcome (muscle density, CSA, cLEMS) is most strongly associated with vBMD and bone structure may inform modifiable targets for improving bone quality and reducing fracture risk after chronic SCI.
The primary objective of this secondary analysis was to examine the associations between pQCTderived calf muscle density and trabecular vBMD at the tibia among adults with chronic SCI. The secondary objective was to examine the associations between calf muscle density, CSA, and function and tibial vBMD, cortical CSA and thickness, and polar moment of inertia (PMI). First, we hypothesize that calf muscle density will be a positive correlate of trabecular and cortical vBMD, cortical CSA and thickness, and PMI at the tibia in individuals with chronic SCI. Second, we hypothesize that of the key muscle variables (cLEMS, CSA and density), calf muscle density and cLEMS will be most strongly associated with trabecular vBMD, whereas calf muscle CSA will be most strongly associated with cortical CSA and PMI.
Materials and Methods
Study design and data collection
A secondary analysis of baseline data from a prospective, observational cohort study was conducted.41 The prospective, observational cohort study was designed to examine a number of primary and secondary research questions related to musculoskeletal health in individuals with chronic SCI. Seventy participants (50 men and 20 women) with SCI (C2-T12, American Spinal Injury Association Impairment Scale [AIS] A-D) were included. Indices of bone quality and muscle density and CSA were determined using pQCT scans of the distal lower extremity (4% and 66% tibia length, respectively). Past and current medical history, demographic characteristics, lifestyle, and impairment data were obtained via participant interview and chart abstraction. Neurological level of injury and AIS classification of participants were determined by a physiatrist using the International Standards for Neurologic Classification of SCI. Duration of injury (years) was calculated as the date of injury minus the date of the baseline assessment. To isolate the effect of voluntary muscle activation on muscle status, the cLEMS of the scanned limb (ankle dorsiflexors, long toe extensors, and plantar flexors) was used in the analyses. Supine height (cm), body mass (kg), and waist circumference (cm) were measured according to SCI-specific anthropometric measurement protocols previously published.41,42 The proportion of participants who met the body mass index (BMI) (≥22 kg/m2) and waist circumference (≥94 cm) criteria for SCIspecific definitions of obesity were determined.43,44 Mobility status was dichotomously classified as using or not using a wheelchair for community mobility. History of tobacco use and co-morbidities were assessed via a subset of questions from the Canadian Multicentre Osteoporosis Study medical history questionnaire.45 The CAGE questionnaire was used to determine alcohol use.46
Participants
Eligible participants were 18 years of age or older, had a traumatic SCI (C2-T12, AIS A-D), were able to provide informed consent, and were able to understand instructions in English. Participants were at least 2 years post injury prior to enrollment. Exclusion criteria were (a) current or prior conditions other than paralysis known to adversely influence bone metabolism, including metabolic disorders, chronic alcoholism, oral glucocorticoids use ≥7.5 mg/day for 3 months or longer, malignancy, and known liver, kidney, or intestinal disease; (b) contraindications to pQCT testing, including bilateral lower extremity metal implants or severe hip and knee flexion contractures; and (c) women who were pregnant or planning to become pregnant. Bisphosphonate exposure was recorded but was not an exclusion criterion. Recruitment strategies were described in a previous publication.41 Written informed consent was obtained from all participants prior to the conduct of formal assessments to determine study eligibility. The current study was approved by the University Health Network and University of Waterloo research ethics boards.
Acquisition and analysis of indices of bone quality using pQCT
Bone quality and muscle density and CSA variables were measured using a pQCT scanner (XCT-2000; Stratec Medizintechnik, Germany), which acquired a transaxial image from 145 projection scans by a narrow fan beam emitted from an x-ray tube. The right tibia was scanned, except in participants with severe lower spasticity or contractures or those with a calf girth that exceeded the gantry opening or those with hardware or prior trauma in the region of interest. Where feasible, the left tibia was scanned as an alternate. Our protocol for pQCT acquisition and analysis has been described in a previous publication.41 Single 2.5-mm slices were completed at distal tibia (4% tibia length) and proximal one-third tibia (66% tibia length). A voxel size of 0.2 mm was used at the 4% site and 0.5 mm was used at the 66% site. pQCT scans from 5 participants were not obtained because of the following reasons: missed appointment (n = 2), died after study enrollment but before pQCT scan acquisition (n = 1), or had severe lower extremity spasticity or a calf circumference that exceeded the size of the gantry opening (n = 2).
Trabecular vBMD (mg/cm3) was determined at the 4% tibia site. Cortical vBMD (mg/cm3), cortical thickness (mm), cortical CSA (mm2), and PMI (mm4) were determined at the 66% tibia sites. These measurement sites are consistent with standard protocols for measuring vBMD and bone structure using pQCT. Trabecular vBMD at the distal tibia was chosen as the primary outcome because accelerated bone loss and fractures often occur at skeletal sites with a higher proportion of trabecular bone.14 Precision for this technique has been reported in individuals with and without SCI.47 The precision (in root mean square coefficient of variation) was 2% or less for all BMD and geometric variables.47 Analyses of the pQCT scans were performed using the manufacturer’s software (Stratec XCT-2000, version 6.00) that applied an iterative contour detection algorithm.48
Muscle density (mg/cm3) and CSA (cm2) were calculated from pQCT scans at the 66% site of the tibial shaft.49,50 This site was chosen because it is the region of the calf with the largest muscle CSA and circumference.50 Images have a slice width of 2.5 mm and voxel size of 0.5 mm. Tissue segmentation and the calculation of muscle density and CSA were performed using SliceOmatic software version 4.3 for PC (SliceOMatic; Tomovision, Montreal, Canada). Manual watershed segmentation of muscle from bone was performed using SliceOmatic V4.3 (Tomovision, Montreal, Canada).3,39 The analysis protocol to determine muscle density and CSA and precision for this technique was previously reported.39 Muscle CSA was adjusted for tibia length in meters (cm2m) in all analyses to provide a more relevant surrogate measure of muscle force-generating potential on bone and correct for differences in muscle length among participants.13,50
Statistical analyses
Descriptive analyses of demographic and clinical characteristics, indices of bone quality, and muscle outcomes were expressed as mean ± standard deviation (SD) for continuous variables and number (%) for categorical variables. Secondary analyses using baseline data from this cohort study have been previously published.3,13,41,51 Independent samples t tests were performed to compare bone quality and muscle outcomes in participants with chronic SCI grouped by sex (men vs women) and AIS classification (AIS A/B vs AIS C/D). Pearson’s correlations were performed to determine the strength of the associations between muscle density and indices of bone quality. Bivariate and multivariable linear regression analyses were performed to determine potential correlates of bone quality outcomes. We examined the following potential confounding variables known to affect BMD and bone microarchitecture in individuals with SCI: AIS classification (motor complete or incomplete; AIS A/B vs AIS C/D), duration of injury (years), sex (male/female), and community wheelchair use (yes/no). We previously demonstrated that history of bisphosphonate use was not associated with bone quality indices in this cohort of 70 individuals with chronic SCI.41 Therefore, we did not control for history of bisphosphonate use in our models. Separate models examining the associations between muscle density, CSA, and function and bone quality indices (trabecular vBMD, cortical vBMD, CSA, and thickness, and PMI) were created for the entire sample. Muscle outcomes (muscle density, CSA, and cLEMS) and confounding variables found to be significant at P < .20 in bivariate regression analyses were entered into a multivariable linear regression model. Sex and duration of injury were controlled for in all models. AIS classification was not entered in the same models as cLEMS due to risk of collinearity. A minimum of 10 observations for each independent variable were included to avoid over-fitting the models.52 The estimate of the coefficients, 95% confidence intervals, P values, and R2 values were reported for the bivariate and multivariable analyses. Regression diagnostic statistics were conducted to assess bias in the models and check for influential cases using standardized residuals and DFBeta values.53 Multicollinearity was assessed using variance inflation factor. The P values were reported to 3 decimal places. Analyses were performed using IBM SPSS version 22.0 (IBM, Armonk, NY).
Results
Description of study participants
The study sample included 70 adults with chronic SCI (50 men and 20 women). The mean ± SD age of participants was 48.8 ± 11.5 years with a mean ± SD duration of injury of 15.5 ± 10 years and LEMS of 11.0 ± 15.7. Participants had a mean ± SD height of 174.5 ± 10.3 cm and weight of 80.1 ± 18.4 kg with a BMI of 26.3 ± 5.6 kg/m2 and waist circumference of 97.4 ± 14.8 cm. Thirty-two (49.2%) and 35 (out of 63; 55.6%) participants met the criteria for BMI and waist circumference SCI-specific definitions of obesity, respectively. Forty-five (64.3%) and 25 (35.7%) participants met impairment criteria for AIS A/B and AIS C/D, respectively. Fifty-one (72.9%) participants had been exposed to bisphosphonate therapy, defined as current or past bisphosphonate use for at least 6 months. Fifty-seven (81.4%) and 61 (87.1%) participants reported a history of calcium and vitamin D supplementation, respectively. Forty (57.0%) and 43 (61.4%) participants had a history of smoking and alcohol use, respectively. Nineteen (27.1%) participants had a history of fragility or low-trauma fracture. Descriptive characteristics of the study participants are shown in Table 1. Table 2 summarizes the mean ± SD for indices of bone quality and muscle density, CSA, and function in participants with SCI grouped by sex (men vs women) and impairment severity (AIS A/B vs AIS C/D).
Table 1. Descriptive characteristics of study participants with chronic SCI (n = 70).
| Characteristics | All participants | Range |
| Demographic characteristics | ||
| Sex, n (%) | ||
| Male | 50 (71.4) | — |
| Female | 20 (28.6) | — |
| Age, mean (SD), years | 48.8 (11.5) | 24–77 |
| Height, mean (SD), cm | 174.5 (10.3) | 140–193 |
| Weight, mean (SD), kg | 80.1 (18.4) | 48.1–137.4 |
| BMI, mean (SD), kg/m2 | 26.3 (5.6) | 16.6–41.6 |
| Waist circumferencea, mean (SD), cm | 97.4 (14.8) | 69.9–148.0 |
| Impairment and mobility characteristics | ||
| Time post injury, mean (SD), years | 15.5 (10.0) | 2–41 |
| Age at injury, mean (SD), years | 33.7 (14.7) | 14–66 |
| Impairment group, n (%) | ||
| Paraplegia AIS A/B | 23 (32.9%) | — |
| Paraplegia AIS C/D | 11 (15.7%) | — |
| Tetraplegia AIS A/B | 22 (31.4%) | — |
| Tetraplegia AIS C/D | 14 (20.0%) | — |
| LEMS, mean (SD) | 11.0 (15.7) | 0–48 |
| Wheelchair use, n (%) | 54 (83.1) | — |
| Bone health and lifestyle characteristics | ||
| History of fragility fracture, n (%) | 19 (27.1) | — |
| Bisphosphonate exposureb, n (%) | 51 (72.9) | — |
| Past calcium supplement usec, n (%) | 57 (81.4) | — |
| Past vitamin D supplement use, n (%) | 61 (87.1) | — |
| History of smoking, n (%) | 40 (57.0) | — |
| History of alcohol usec, n (%) | 43 (61.4) | — |
Note: AIS = American Spinal Injury Association Impairment Scale; BMI = body mass index; LEMS = lower extremity motor score; SCI = spinal cord injury; SD = standard deviation.
Waist circumference (n = 68).
Bisphosphonate exposure is defined as current use or past history of bisphosphonate medications (including alendronate [Fosamax/Fosavance], risedronate [Actonel/Actonel Delayed Release], zoledronate [Aclasta], etidronate [Didrocal], and pamidronate [Aredia]).
Past calcium supplement use was unknown for one participant. Three participants did not respond regarding history of alcohol use.
Table 2. Indices of bone quality and muscle density, CSA, and function in participants with chronic SCI grouped by sex and impairment severity (n = 65a).
| Anatomic site |
Variable (units) |
All participants with chronic SCI (n = 65a) |
Male participants with chronic SCI (n = 45) |
Female participants with chronic SCI (n = 20) |
Participants with AIS A/B (n = 40) |
Participants with AIS C/D (n = 25) |
| 4% tibia | Trabecular vBMD (mg/cm3) | 130.1 (54.5) | 137.2 (55.3) | 114.1 (50.3) | 101.0 (38.7) | 176.7 (42.6)d |
| 66% tibia | Cortical vBMD (mg/cm3) | 1,082.1 (51.8) | 1,179.8 (53.7) | 1,137.6 (66.4)c | 1,139.1 (73.0) | 1,168.8 (46.7) |
| Cortical thickness (mm) | 3.21 (0.94) | 3.37 (0.95) | 2.84 (0.83)c | 2.84 (0.92) | 3.80 (0.65)d | |
| Cortical CSA (mm2) | 256.0 (76.7) | 278.8 (74.9) | 204.7 (53.4)c | 228.5 (67.5) | 300.0 (70.8)d | |
| PMI (mm4) | 45,304 (18,602) | 52,188 (17,505) | 29,816 (9,551)c | 40,867 (15,750) | 52,404 (20,840)d | |
| Muscle density (mg/cm3) | 53.5 (13.3) | 54.2 (13.6) | 51.9 (12.7) | 49.4 (13.8) | 60.2 (9.3)d | |
| Muscle CSAb (cm2m) | 19.9 (8.7) | 22.1 (8.7) | 14.9 (6.5)c | 16.3 (6.8) | 25.7 (8.3)d | |
| cLEMS | 2.8 (4.7) | 3.09 (4.97) | 2.05 (4.20) | 0 (0) | 7.2 (5.2)d |
Note: Values given as mean (SD). SCI = spinal cord injury; AIS = American Spinal Injury Association Impairment Scale; cLEMS = calf lower extremity motor score; CSA = cross-sectional area; pQCT = peripheral quantitative computed tomography; SD = standard deviation; vBMD = volumetric bone mineral density.
Five participants did not complete pQCT scans.
Muscle CSA (cm2) was multiplied by tibia length (m).
Significant difference between male and female participants (P < .05).
Significant difference between AIS A/B and AIS C/D groups (P < .05).
Bivariate and multivariable regression analyses of muscle-bone associations
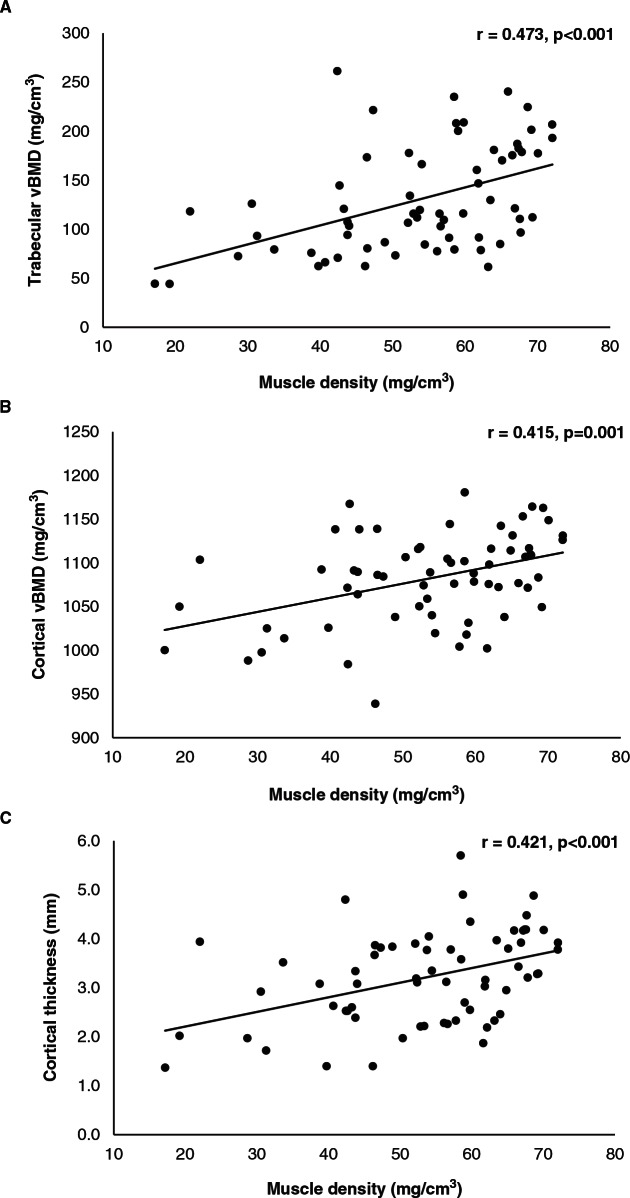
Moderately strong positive associations were observed between muscle density and trabecular vBMD (r = 0.473, P < .001), cortical vBMD (r = 0.415, P = .001), and cortical thickness (r = 0.421, P < .001) (Figure 1), whereas weak-to-moderate positive associations were observed between muscle density and cortical CSA (r = 0.367, P = .003) and PMI (r = 0.245, P = .049).
Figure 1.
Pearson’s correlations were performed to determine the strength of the associations between muscle density and indices of bone quality in our participants with chronic spinal cord injury. Moderately strong associations were observed between muscle density and trabecular volumetric bone mineral density (vBMD) (r = 0.473, P < .001), cortical vBMD (r = 0.415, P < .001), and cortical thickness (r = 0.421, P < .001).
Results of the bivariate and multivariable regression analyses of muscle-bone associations are presented in Table 3. Muscle density was positively associated with trabecular vBMD (b = 1.94 [1.03, 2.85]), cortical vBMD (b = 1.62 [0.72, 2.51]), cortical thickness (b = 0.03 [0.01, 0.05]), cortical CSA (b = 2.12 [0.77, 3.47]), and PMI (b = 342.6 [1.30, 683.9]) (P < .05). Muscle CSA and cLEMS were positively associated with all indices of bone quality (trabecular vBMD, cortical thickness, cortical CSA, and PMI) (P ≤ .001), except for cortical vBMD (P > .05). After adjusting for sex, duration of injury, AIS classification, and wheelchair use, muscle density remained a significant positive correlate of trabecular vBMD (b = 0.85 [0.04, 1.66]), cortical thickness (b = 0.02 [0.001, 0.034]), and cortical vBMD (b = 1.70 [0.71, 2.69]) (P < .05), whereas muscle CSA was a significant positive correlate of cortical CSA (b = 2.50 [0.12, 4.88]) and PMI (b = 731.8 [161.7, 1301.9]) (P < .05). Muscle CSA was not significantly associated with trabecular vBMD and cortical thickness after adjusting for confounding variables (P > .05). After adjusting for sex, duration of injury, and wheelchair use, cLEMS remained a significant positive correlate of trabecular vBMD (b = 7.69 [4.63, 10.76]), cortical CSA (b = 5.85 [0.27, 11.43]), and cortical thickness (b = 0.10 [0.03, 0.16]) (P < .05).
Table 3. Bivariate and multivariable regression analyses of muscle-bone associations in participants with chronic SCI (n = 65a).
| Unadjusted muscle-bone associations | Muscle-bone associations adjusted for confounding variables | |||||
| b (95% CI) | P value | R2 | b (95% CI) | P value | R2 | |
| Trabecular vBMD, mg/cm3 | ||||||
| Muscle density | 1.94 (1.03, 2.85) |
<.001 | 0.223 | 0.85 (0.04, 1.66)c |
<.040 | 0.544 |
| Muscle CSAb | 3.46 (2.14, 4.78) |
<.001 | 0.304 | 1.29 (−0.28, 2.86)c |
.105 | 0.532 |
| cLEMS | 7.80 (5.67, 9.93) |
<.001 | 0.459 | 7.69 (4.63, 10.76)d |
<.001 | 0.503 |
| Cortical vBMD, mg/cm3 | ||||||
| Muscle density | 1.62 (0.72, 2.51) |
<.001 | 0.172 | 1.70 (0.71, 2.69)c |
<.001 | 0.248 |
| Muscle CSAb | 0.79 (-0.70, 2.28) |
.294 | 0.017 | 0.88 (-1.17, 2.94)c |
.394 | 0.109 |
| cLEMS | 2.40 (-0.29, 5.08) |
.079 | 0.048 | 2.77 (-1.14, 6.68)d |
.161 | 0.104 |
| Cortical thickness, mm | ||||||
| Muscle density | 0.03 (0.01, 0.05) |
<.001 | 0.177 | 0.02 (0.001, 0.034)c |
.042 | 0.370 |
| Muscle CSAb | 0.05 (0.03, 0.07) |
<.001 | 0.210 | 0.02 (-0.01, 0.05)c |
.195 | 0.343 |
| cLEMS | 0.10 (0.05, 0.14) |
<.001 | 0.233 | 0.10 (0.03, 0.16)d |
.003 | 0.313 |
| Cortical CSA, mm2 | ||||||
| Muscle density | 2.12 (0.77, 3.47) |
.003 | 0.135 | 0.98 (-0.29, 2.25)c |
.127 | 0.438 |
| Muscle CSAb | 4.94 (3.10, 6.78) |
<.001 | 0.313 | 2.50 (0.12, 4.88)c |
.040 | 0.456 |
| cLEMS | 8.12 (4.59, 11.65) |
<.001 | 0.251 | 5.85 (0.27, 11.43)d |
.040 | 0.456 |
| PMI, mm4 | ||||||
| Muscle density | 342.6 (1.30, 683.9) |
.049 | 0.060 | 107.7 (-206.1, 421.47)c |
.495 | 0.414 |
| Muscle CSAb | 1205.8 (760.2, 1651.3) |
<.001 | 0.317 | 731.8 (161.7, 1301.9)c |
.013 | 0.469 |
| cLEMS | 1611.6 (709.8, 2513.4) |
.001 | 0.168 | 1412.0 (311.3, 2512.6)d |
.013 | 0.449 |
Note: AIS = American Spinal Injury Association Impairment Scale (AIS); cLEMS = calf lower extremity motor score; CSA = cross-sectional area; SCI = spinal cord injury; vBMD = volumetric bone mineral density. Boldface P values indicate statistical significance (P < .05).
Five participants did not complete pQCT scans.
Muscle CSA (cm2) was multiplied by tibia length (m).
Adjusted model for sex, duration of injury, AIS classification (AIS A/B vs AIS C/D), and wheelchair use.
Adjusted model for sex, duration of injury, and wheelchair use.
Discussion
Summary of findings
Consistent with our hypothesis, we observed moderately strong positive associations between calf muscle density and trabecular and cortical vBMD and cortical thickness at the tibia in adults with chronic SCI. However, we found weak to nonexistent positive associations between calf muscle density and cortical CSA and PMI at the tibial shaft after adjusting for relevant confounding variables. Alternatively, we demonstrated that calf muscle CSA was a stronger positive correlate of cortical CSA and PMI at the tibial shaft in individuals with chronic SCI than calf muscle density. As such, our findings suggest calf muscle density may exert a greater influence on tibial vBMD and cortical thickness in individuals with chronic SCI than calf muscle CSA. In addition, cLEMS was most strongly associated with trabecular vBMD at the distal tibia consistent with previous findings in individuals with chronic SCI.13 The current study contributes to a growing body of research on the muscle-bone unit theory under conditions of disuse and neurological impairment and provides data on the contributions of muscle density to bone quality indices at the tibia.13,40 Thus, changes in tibial vBMD and cortical thickness over time following SCI may be more closely linked to calf muscle density and function, whereas changes in tibial bone size and geometry following SCI may be more closely linked to calf muscle size.
Association between muscle density and bone quality
Substantial evidence links elevated muscle adiposity in the leg and impaired metabolic status (eg, decreased insulin sensitivity, muscle mitochondrial dysfunction) in individuals with SCI.1,16 However, the associations between calf muscle density (a surrogate of fatty infiltration when evaluating the muscle-bone unit) and lower extremity bone quality under conditions of disuse and neurological impairment are not as well understood. In a cross-sectional study of stroke survivors with subacute lower limb hemiparesis, MacIntyre et al54 observed a moderately strong association between muscle density and cortical vBMD at the tibia. Similar to stroke, sublesional adiposity following SCI is attributed to persistent mobility limitation and neuromuscular impairment in the paralyzed limbs. We demonstrated that muscle density was a moderately strong positive correlate of indices of bone quality at the tibia, including trabecular and cortical vBMD, and cortical thickness, after adjusting for confounding variables known to influence vBMD and bone microarchitecture. Our findings suggest that lower muscle density or fatty infiltration at the calf is linked to lower vBMD at the distal lower extremity among individuals with chronic SCI. However, changes in muscle density in larger muscle groups (eg, quadriceps) may assert different or more potent effects on lower extremity bone quality (eg, femur) following SCI.55
Associations between muscle CSA and function and bone quality
A unique finding of the current study is that muscle CSA was a stronger correlate of cortical CSA and PMI than muscle density. Conflicting evidence was recently published40 suggesting that the association between muscle and bone outcomes was weak in adults with complete SCI paraplegia due to the absence of voluntary muscle contraction. Alternatively, Totosy de Zepetnek et al13 demonstrated that associations between muscle CSA and function and indices of bone quality were significant among individuals with chronic SCI and remained significant after adjusting for duration of injury. Further, Totosy de Zepetnek et al13 observed a stronger association between cLEMS and trabecular vBMD at the distal tibia compared to muscle CSA, supporting the importance of muscle function as a possible target for intervention after injury. Similarly, we found moderately strong positive associations between cLEMS and trabecular vBMD, cortical thickness, and cortical CSA. Our findings suggest that muscle size and function may represent therapeutic targets for preventing bone loss and attenuating fracture risk following SCI, in addition to being related to glucose metabolism and muscle strength.2,11,13,55
Study strengths and limitations
Strengths of this study include its diverse, large cohort of adult men and women with chronic motor complete and incomplete SCI and the multivariable regression analysis of muscle-bone associations controlled for relevant confounders. However, the cohort with chronic SCI included a large proportion of individuals who were 15 years or more post injury. A broader distribution of duration of injury might elucidate different muscle-bone relationships of varying strength and significance over time in individuals with SCI. Further, the findings regarding muscle-bone associations may be influenced by the active or remote exposure to bisphosphonate therapy in our participants with chronic SCI. A large proportion of participants (>70%) were current or past bisphosphonate users, primarily to treat low BMD by preventing declines in bone mass or attenuating bone resorption. Also, the nature of the crosssectional design limits our ability to make causal inferences from the data regarding the musclebone unit theory in response to SCI.
Resolution limitations with pQCT analysis affected our ability to differentiate between soft and adipose or noncontractile tissues contained within the fascia border of the calf muscle group in participants with extreme muscle atrophy. Our protocol may have overestimated calf muscle CSA by failing to account for the contributions of intra- and intermuscular adipose stores.1 Magnetic resonance imaging is a more sensitive technique for measuring fatty infiltration of skeletal muscle across the entire length of the leg,33 yet recent evidence demonstrates that pQCT-derived muscle density of the leg may represent a valid proxy for intra- and intermuscular fat.39,56 There were several technical challenges related to pQCT acquisition in the cohort of individuals with SCI that precluded accurate analysis, including lower leg edema, spasticity, and calf girths that exceeded the size of the pQCT gantry. Also, we did not measure and were unable to control for metabolic biomarkers that may explain greater variability in the association between muscle density and bone quality outcomes, particularly in individuals with SCI.
Conclusions and future directions
Our findings demonstrate that calf muscle density and cLEMS were more strongly associated with trabecular vBMD at the tibia in adults with chronic SCI than calf muscle size, evolving our understanding of the muscle-bone unit theory as it relates to SCI. Alternatively, muscle size represented an important determinant of tibial bone size and geometry in individuals with chronic SCI. Because changes in muscle precede changes in bone in the subacute phases of injury, muscle density, size, and function may represent modifiable targets for rehabilitation interventions with the goal of improving bone quality and reducing fracture risk.57 Future research should examine the effect of therapeutic interventions (eg, electrical stimulation, vibration, resistance training) targeting muscle density, size, and function on longitudinal changes in tibial vBMD and bone microarchitecture and fracture risk after chronic SCI.
Acknowledgments
Conflicts of interest: Dr. Craven reports grants from the Canadian Institutes of Health Research (CIHR) and from the Rick Hansen Institute (RHI) during the conduct of the study. Dr. Craven also acknowledges funding from the Toronto Rehabilitation Institute and UHNRMA AFP Innovation funds. Dr. Adachi acts as a consultant for Amgen, Eli Lilly, and Merck. He has received honoraria from Amgen, Eli Lilly, and Merck and has received research funding from Amgen and Eli Lilly. McMaster University has received funding from Actavis to complete a chair in rheumatology. Dr. Giangregorio is the recipient of a CIHR New Investigator Award and an Early Researcher Award from the Ontario Ministry of Research and Innovation. The other authors have nothing to disclose.
Financial support: The Bone Quality in Canadians with Chronic SCI Cohort Study was funded by the Canadian Institutes of Health Research (CIHR) (grant 86521), ONF-REPAR (ONF operating grant 2011-ONF-REPAR2-885), and the Rick Hansen Foundation (grant 2011-15S-RES3-tri-100812). The infrastructure was funded by the Ontario Research Fund and the Canadian Foundation for Innovation.
Adherence to ethics and reporting requirements: Approved by the University Health Network research ethics board (REB #08-027) and University of Waterloo research ethics board (ORE #19399).
Additional contributions: We gratefully acknowledge the contributions of Maggie Szeto, Louise Brisbois, Lindsie Robertson, and Cheryl Lynch at the Lyndhurst Centre for assistance with data collection, data entry and cleaning, REB correspondence, and subject retention. We are appreciative of the contributions of Alexandra Papaioannou, Milos Popovic, and Neil McCartney to the grant proposal and study design. We wish to thank Lesley Beaumont, Rod Rhem, Christopher Gordon, Angela Cheung, Marta Erlandson, Deena Lala, and Eva Szabo for their assistance with pQCT acquisition and analysis. We also would like to acknowledge Andy Kin On Wong for developing and sharing the SliceOmatic tissue segmentation process. Finally, we would like to extend our sincerest gratitude to all of the participants for their generosity and time commitment to the study.
References
- 1. Gorgey AS, Dudley GA. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007;45(4):304–309. [DOI] [PubMed] [Google Scholar]
- 2. Shah PK, Stevens JE, Gregory CM, et al. Lowerextremity muscle cross-sectional area after incomplete spinal cord injury. Arch Phys Med Rehabil. 2006;87(6):772–778. [DOI] [PubMed] [Google Scholar]
- 3. Moore CD, Craven BC, Thabane L, et al. Lowerextremity muscle atrophy and fat infiltration after chronic spinal cord injury. J Musculoskelet Neuronal Interact. 2015;15(1):32–41. [PMC free article] [PubMed] [Google Scholar]
- 4. Castro MJ, Apple DF, Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80(4):373–378. [DOI] [PubMed] [Google Scholar]
- 5. Talmadge RJ, Castro MJ, Apple DF, Jr, Dudley GA. Phenotypic adaptations in human muscle fibers 6 and 24 wk after spinal cord injury. J Appl Physiol (1985). 2002;92(1):147–154. [DOI] [PubMed] [Google Scholar]
- 6. Garland DE, Adkins RH. Bone loss at the knee in spinal cord injury. Top Spinal Cord Inj Rehabil. 2001;6(3):37–46. [Google Scholar]
- 7. Garland DE, Adkins RH, Kushwaha V, Stewart C. Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med. 2004;27(3):202–206. [DOI] [PubMed] [Google Scholar]
- 8. Dauty M, Perrouin Verbe B, Maugars Y, Dubois C, Mathe JF. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone. 2000;27(2):305–309. [DOI] [PubMed] [Google Scholar]
- 9. Spungen AM, Adkins RH, Stewart CA, et al. Factors influencing body composition in persons with spinal cord injury: A cross-sectional study. J Appl Physiol (1985). 2003;95(6):2398–2407. [DOI] [PubMed] [Google Scholar]
- 10. Spungen AM, Wang J, Pierson RN, Jr., Bauman WA. Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J Appl Physiol (1985). 2000;88(4):1310–1315. [DOI] [PubMed] [Google Scholar]
- 11. Eser P, Frotzler A, Zehnder Y, Schiessl H, Denoth J. Assessment of anthropometric, systemic, and lifestyle factors influencing bone status in the legs of spinal cord injured individuals. Osteoporos Int. 2005;16(1):26–34. [DOI] [PubMed] [Google Scholar]
- 12. Giangregorio LM, Craven BC, Webber CE. Musculoskeletal changes in women with spinal cord injury: A twin study. J Clin Densitom. 2005;8(3): 347–351. [DOI] [PubMed] [Google Scholar]
- 13. Totosy de Zepetnek JO, Craven BC, Giangregorio LM. An evaluation of the muscle-bone unit theory among individuals with chronic spinal cord injury. Spinal Cord. 2012;50(2):147–152. [DOI] [PubMed] [Google Scholar]
- 14. Eser P, Frotzler A, Zehnder Y, Denoth J. Fracture threshold in the femur and tibia of people with spinal cord injury as determined by peripheral quantitative computed tomography. Arch Phys Med Rehabil. 2005;86(3):498–504. [DOI] [PubMed] [Google Scholar]
- 15. Bauman WA, Spungen AM. Metabolic changes in persons after spinal cord injury. Phys Med Rehabil Clin N Am. 2000;11(1):109–140. [PubMed] [Google Scholar]
- 16. Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA. Intramuscular fat and glucose tolerance after spinal cord injury--a cross-sectional study. Spinal Cord. 2004;42(12):711–716. [DOI] [PubMed] [Google Scholar]
- 17. Wahman K, Nash MS, Westgren N, Lewis JE, Seiger A, Levi R. Cardiovascular disease risk factors in persons with paraplegia: The Stockholm spinal cord injury study. J Rehabil Med. 2010;42(3):272–278. [DOI] [PubMed] [Google Scholar]
- 18. Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord. 2001;39(4):208–214. [DOI] [PubMed] [Google Scholar]
- 19. Ragnarsson KT, Sell GH. Lower extremity fractures after spinal cord injury: A retrospective study. Arch Phys Med Rehabil. 1981;62(9):418–423. [PubMed] [Google Scholar]
- 20. Freehafer AA, Hazel CM, Becker CL. Lower extremity fractures in patients with spinal cord injury. Paraplegia. 1981;19(6):367–372. [DOI] [PubMed] [Google Scholar]
- 21. Morse LR, Battaglino RA, Stolzmann KL, et al. Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporos Int. 2009;20(3):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carbone LD, Chin AS, Burns SP, et al. Morbidity following lower extremity fractures in men with spinal cord injury. Osteoporos Int. 2013;24(8):2261–2267. [DOI] [PubMed] [Google Scholar]
- 23. Phonthee S, Saengsuwan J, Siritaratiwat W, Amatachaya S. Incidence and factors associated with falls in independent ambulatory individuals with spinal cord injury: A 6-month prospective study. Phys Ther. 2013;93(8):1061–1072. [DOI] [PubMed] [Google Scholar]
- 24. Migliorini CE, New PW, Tonge BJ. Quality of life in adults with spinal cord injury living in the community. Spinal Cord. 2011;49(3):365–370. [DOI] [PubMed] [Google Scholar]
- 25. Noonan VK, Fingas M, Farry A, et al. Incidence and prevalence of spinal cord injury in Canada: A national perspective. Neuroepidemiology. 2012;38(4): 219–226. [DOI] [PubMed] [Google Scholar]
- 26. Zehnder Y, Luthi M, Michel D, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: A cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004;15(3):180–189. [DOI] [PubMed] [Google Scholar]
- 27. Frost HM. Bone “mass” and the “mechanostat”: A proposal. Anat Rec. 1987;219(1):1–9. [DOI] [PubMed] [Google Scholar]
- 28. Schoenau E. From mechanostat theory to development of the “Functional Muscle-Bone-Unit.” J Musculoskelet Neuronal Interact. 2005;5(3):232–238. [PubMed] [Google Scholar]
- 29. Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995;33(11):674–677. [DOI] [PubMed] [Google Scholar]
- 30. Frey-Rindova P, de Bruin ED, Stussi E, Dambacher MA, Dietz V. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord. 2000;38(1):26–32. [DOI] [PubMed] [Google Scholar]
- 31. de Bruin ED, Vanwanseele B, Dambacher MA, Dietz V, Stussi E. Long-term changes in the tibia and radius bone mineral density following spinal cord injury. Spinal Cord. 2005;43(2):96–101. [DOI] [PubMed] [Google Scholar]
- 32. Biering-Sorensen F, Bohr HH, Schaadt OP. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest. 1990;20(3):330–335. [DOI] [PubMed] [Google Scholar]
- 33. Gorgey AS, Poarch HJ, Adler RA, Khalil RE, Gater DR. Femoral bone marrow adiposity and cortical bone cross-sectional areas in men with motor complete spinal cord injury. PM R. 2013;5(11):939–948. [DOI] [PubMed] [Google Scholar]
- 34. Bauman WA, Spungen AM, Wang J, Pierson RN, Jr, Schwartz E. Relationship of fat mass and serum estradiol with lower extremity bone in persons with chronic spinal cord injury. Am J Physiol Endocrinol Metab. 2006;290(6):E1098–1103. [DOI] [PubMed] [Google Scholar]
- 35. Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90(6):1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marcus RL, Addison O, Dibble LE, Foreman KB, Morrell G, Lastayo P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J Aging Res. 2012;2012:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shah PK, Gregory CM, Stevens JE, et al. Non-invasive assessment of lower extremity muscle composition after incomplete spinal cord injury. Spinal Cord. 2008;46(8):565–570. [DOI] [PubMed] [Google Scholar]
- 38. Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985). 2001;90(6):2157–2165. [DOI] [PubMed] [Google Scholar]
- 39. Wong AK, Hummel K, Moore C, et al. Improving reliability of pQCT-derived muscle area and density measures using a watershed algorithm for muscle and fat segmentation. J Clin Densitom. 2015;18(1): 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dionyssiotis Y, Stathopoulos K, Trovas G, Papaioannou N, Skarantavos G, Papagelopoulos P. Impact on bone and muscle area after spinal cord injury. Bonekey Rep. 2015;4:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lala D, Craven BC, Thabane L, et al. Exploring the determinants of fracture risk among individuals with spinal cord injury. Osteoporos Int. 2014;25(1): 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Edwards LA, Bugaresti JM, Buchholz AC. Visceral adipose tissue and the ratio of visceral to subcutaneous adipose tissue are greater in adults with than in those without spinal cord injury, despite matching waist circumferences. Am J Clin Nutr. 2008;87(3): 600–607. [DOI] [PubMed] [Google Scholar]
- 43. Ravensbergen HR, Lear SA, Claydon VE. Waist circumference is the best index for obesity-related cardiovascular disease risk in individuals with spinal cord injury. J Neurotrauma. 2014;31(3):292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Laughton GE, Buchholz AC, Martin Ginis KA, Goy RE, Group SSR. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord. 2009;47(10):757–762. [DOI] [PubMed] [Google Scholar]
- 45. Kreiger N, Tenenhouse A, Joseph L, et al. The Canadian Multicentre Osteoporosis Study (CaMos): Background, rationale, methods. The Canadian Journal of Aging. 1999;18:376–387. [Google Scholar]
- 46. Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252(14):1905–1907. [DOI] [PubMed] [Google Scholar]
- 47. Giangregorio L, Lala D, Hummel K, Gordon C, Craven BC. Measuring apparent trabecular density and bone structure using peripheral quantitative computed tomography at the tibia: Precision in participants with and without spinal cord injury. J Clin Densitom. 2013;16(2):139–146. [DOI] [PubMed] [Google Scholar]
- 48. Ashe MC, Khan KM, Kontulainen SA, et al. Accuracy of pQCT for evaluating the aged human radius: An ashing, histomorphometry and failure load investigation. Osteoporos Int. 2006;17(8):1241–1251. [DOI] [PubMed] [Google Scholar]
- 49. Schoenau E, Neu CM, Mokov E, Wassmer G, Manz F. Influence of puberty on muscle area and cortical bone area of the forearm in boys and girls. J Clin Endocrinol Metabol. 2000;85(3):1095–1098. [DOI] [PubMed] [Google Scholar]
- 50. Rittweger J, Beller G, Ehrig J, et al. Bone-muscle strength indices for the human lower leg. Bone. 2000;27(2):319–326. [DOI] [PubMed] [Google Scholar]
- 51. Hummel K, Craven BC, Giangregorio L. Serum 25(OH)D, PTH and correlates of suboptimal 25(OH) D levels in persons with chronic spinal cord injury. Spinal Cord. 2012;50(11):812–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Babyak MA. What you see may not be what you get: A brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004;66(3):411–421. [DOI] [PubMed] [Google Scholar]
- 53. Stevens JP. Applied Multivariate Statistics for the Social Sciences. 4th ed. Hillsdale NJ: Erlbaum; 2002. [Google Scholar]
- 54. MacIntyre NJ, Rombough R, Brouwer B. Relationships between calf muscle density and muscle strength, mobility and bone status in the stroke survivors with subacute and chronic lower limb hemiparesis. J Musculoskelet Neuronal Interact. 2010;10(4): 249–255. [PubMed] [Google Scholar]
- 55. Modlesky CM, Slade JM, Bickel CS, Meyer RA, Dudley GA. Deteriorated geometric structure and strength of the midfemur in men with complete spinal cord injury. Bone. 2005;36(2):331–339. [DOI] [PubMed] [Google Scholar]
- 56. Wong AK, Beattie KA, Min KK, et al. Peripheral quantitative computed tomography-derived muscle density and peripheral magnetic resonance imagingderived muscle adiposity: Precision and associations with fragility fractures in women. J Musculoskelet Neuronal Interact. 2014;14(4):401–410. [PMC free article] [PubMed] [Google Scholar]
- 57. Craven BC, Lynch CL, Eng JJ. Bone health following spinal cord injury. In: Eng JJ, Teasell RW, Miller WC, et al., eds. Spinal Cord Injury Rehabilitation Evidence. Vol 5 Vancouver: SCIRE; 2014:1–37. [Google Scholar]