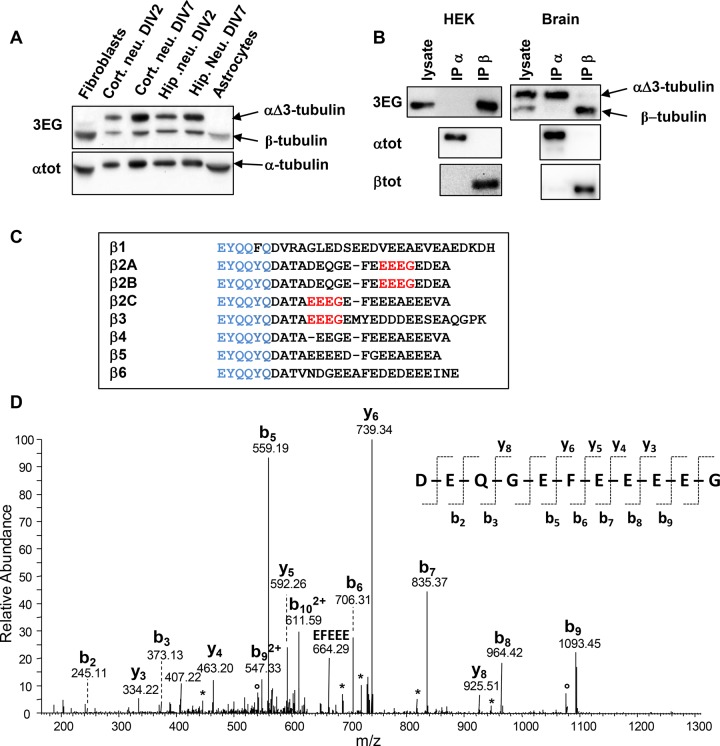
FIGURE 4:
Evidence for a ubiquitous truncated form of β-tubulin. (A) Immunoblot of crude extracts from the indicated mouse tissues (same experiment as Figure 2B, but with the lower protein bands included). Protein levels were controlled using αtot antibody. (B) Immunoprecipitation of endogenous α- and β-tubulins from HEK293T cells and neonate mouse brain using αtot (IP α) and βtot (IP β) antibodies and analysis with 3EG antibody. The quality of immunoprecipitations was controlled using αtot and βtot antibodies together with mouse TrueBlot secondary antibodies. (C) Alignment of C-termini from the eight mouse β-tubulin isotypes. The βtot antibody epitope is in blue, and the 3EG epitope is in red. (D) Identification of the C-terminal peptide of the truncated β2A/B-tubulin (ΔEDEA) from neonatal mouse brain. The MS/MS spectrum of the peptide ion with m/z = 649.24, corresponding to an experimental monoprotonated mass MH+ of 1296.4632 Da, is annotated. The b- and y-type fragments identified are indicated on the sequence of the C-terminal peptide DEQGEFEEEEG, with a theoretical mass MH+ of 1296.4630 Da. Loss of H2O and NH3 is labeled with asterisks and open dots, respectively.