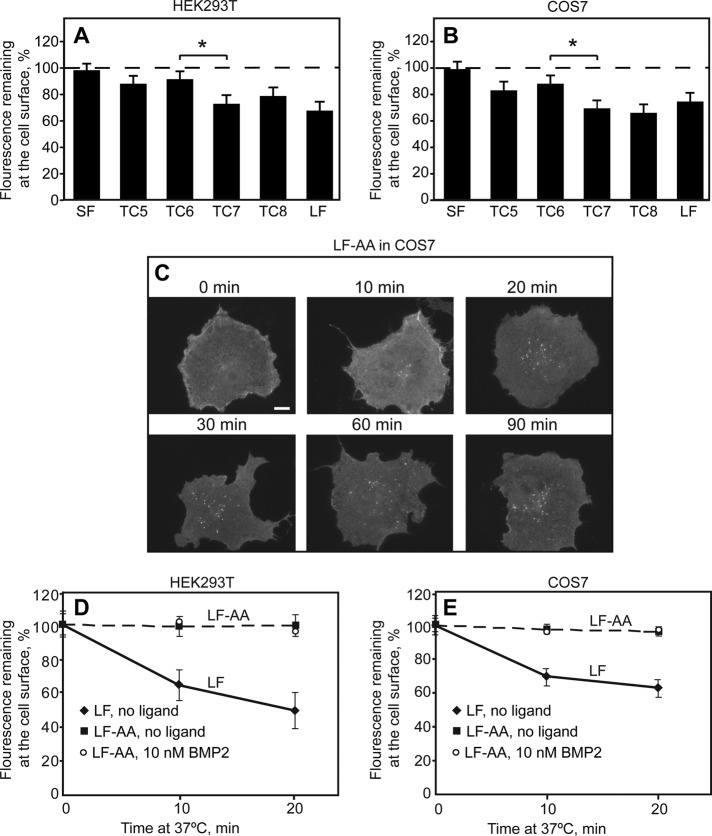
FIGURE 6:
Endocytosis of BMPRII-SF, BMPRII-LF and its truncation and alanine replacement mutants. HEK293T or COS7 cells were transfected with the indicated myc-BMPRII variants, followed by immunofluorescence labeling of the cell surface receptors at 4°C as in Figure 4. Samples were then shifted to 37°C for 20 min (A, B) or for the indicated times (C–E) to allow endocytosis. (A, B) The internalization determinant of BMPRII localizes to the region between TC6 and TC7. For each construct, fluorescence intensity at the cell surface was measured by the point-confocal method (200 cells/sample) at time 0 and after a 20-min incubation at 37°C, and the intensity of the same sample at time 0 was taken as 100%. The asterisk denotes a significant difference between TC6 and TC7 (*p < 0.05). (C) Typical images of cells expressing the endocytosis-defective BMPRII-LF-AA mutant before and after internalization for the indicated periods (10–90 min). Bar, 10 μm. Note the lack of internalization (very little vesicular staining) even at long incubation periods. (D, E) Quantification of myc-BMPRII-LF-AA endocytosis relative to myc-BMPRII-LF in HEK293T (D) and in COS7 (E) cells. The fluorescence intensity remaining at the cell surface was measured by the point-confocal method (Materials and Methods). Results are mean ± SEM of 200 cells/time point, taking for each sample the intensity at time 0 as 100%. As shown in D and E, addition of ligand (10 nM BMP2) as in Figure 5 had no effect on the internalization of BMPRII-LF-AA in either cell line.