Abstract
Rabies is a zoonotic disease caused by the rabies virus. While the salivary glands are important as exit and propagation sites for the rabies virus, the mechanisms of rabies excretion remain unclear. Here, we investigated the histopathology of the salivary glands of rabid dogs and analyzed the mechanism of excretion into the oral cavity. Mandibular and parotid glands of 22 rabid dogs and three control dogs were used. Mild to moderate non-suppurative sialadenitis was observed in the mandibular glands of 19 of the 22 dogs, characterized by loss of acinar epithelium and infiltration by lymphoplasmacytic cells. Viral antigens were detected in the mucous acinar epithelium, ganglion neurons and myoepithelium. Acinar epithelium and lymphocytes were positive for anti-caspase-3 antibodies and TUNEL staining. In contrast, no notable findings were observed in the ductal epithelial cells and serous demilune. In the parotid gland, the acinar cells, myoepithelium and ductal epithelium all tested negative. These findings confirmed the path through which the rabies virus descends along the facial nerve after proliferation in the brain to reach the ganglion neurons of the mandibular gland, subsequently traveling to the acinar epithelium via the salivary gland myoepithelium. Furthermore, the observation that nerve endings passing through the myoepithelium were absent from the ductal system suggested that viral proliferation and cytotoxicity could not occur there, ensuring that secretions containing the virus are efficiently excreted into the oral cavity.
Keywords: histopathology, Philippines, rabid dog, salivary gland
Rabies virus is a highly neurotropic virus that affects the nervous system in humans and animals, resulting in death of the infected individual [14]. Rabies is an endemic disease in many developing and developed countries worldwide and causes approximately 37,000–86,000 human deaths each year [36]. In endemic countries, domestic dogs remain the principal reservoir and vector for rabies virus infection and play an important role in transmission of rabies virus to humans [2, 25]. In addition, more than 98% of human rabies deaths in the Philippines are associated with dog bites [8]. Viral transmission is achieved through contact with the virus contained in the saliva of an infected animal, often through biting. After deep biting by an infected animal, the rabies virus binds to the nicotinic acetylcholine receptors at the neuromuscular junction in the muscle fibers [21]. The virus enters through the peripheral nerves and reaches the central nervous system (CNS) by centripetal spread. The rabies virus then spreads to peripheral non-nervous tissues, including the salivary glands, adrenal glands, gastrointestinal tract, pancreas and heart [7, 12, 16, 33].
The salivary glands are an important site of viral replication and portals of exit for the rabies virus into the saliva [14]. A previous study found that dogs have the ability to excrete virus particles into the saliva for up to 14 days before any clinical symptoms of rabies are apparent [10]. Moreover, saliva samples can be used as alternatives to brain [18] and cerebrospinal fluid [28] samples for ante-mortem diagnosis of canine rabies. The acinar epithelium of salivary glands has been shown to contain abundant rabies virus antigens in infected animals [3, 6, 10]. However, detailed pathological findings in salivary glands and analysis of the excretion mechanism have not been reported.
Therefore, in this study, we investigated the histopathological and immunohistochemical findings of the salivary glands of rabid dogs and evaluated the excretion mechanism of the virus into the oral cavity.
MATERIALS AND METHODS
Animals and direct fluorescent antibody test (dFAT): Mandibular and parotid gland samples were obtained from 22 rabid dogs, which had been submitted to the Research Institute of Tropical Medicine (RITM), Philippines, for postmortem diagnosis of rabies. Small transverse sections (2–3 mm in thickness) of ammon’s horn and medulla were cut, and slide was touched against the cut surface of the section and then placed on cold acetone overnight for fixing. After fixation, slides were air dried at room temperature. Then, 450 µl of fluorescence isothiocyanate conjugate anti-rabies monoclonal antibody (Fujirebio®, Malvern, PA, U.S.A.) was added. The slides were incubated for 30 min at 37°C in a high humidity chamber. Slides were then dipped and rinsed for 20 to 25 times in PBS twice followed by distilled water for further washing. Small amounts of the mounting medium, 20% glycerol-Tris buffered saline pH 9.0, were placed on the slides before covering with coverslips for examination. The slides were examined under the fluorescent microscope (80i, Nikon, Tokyo, Japan).
Histopathological examination: Mandibular and parotid salivary glands of rabid (n=22) and control (n=3) dogs were fixed in 10% neutral buffered formalin at room temperature (RT) for more than 72 hr, embedded in paraffin, sectioned (3 µm thickness) and mounted. Three rabies-vaccinated domestic Japanese mixed dogs (8 to 10 years old) were used as a control group. The sections were then subjected to hematoxylin and eosin (HE), special staining (Alcian blue and reticulin silver impregnation) and immunohistochemistry as described below.
Immunohistochemistry: For detection of rabies virus antigen in tissues, sections were stained using the streptavidin-biotin-peroxidase complex (LSAB) method with anti-rabbit phosphoprotein (P) as described previously [19]. For detection of cell type, the following primary antibodies were used: anti-CD3 for T lymphocytes (DAKO, Kyoto, Japan); anti-CD20 (Spring Bioscience, Fremont, CA, U.S.A.) and CD79α (DAKO); anti-laminin for basement membranes (Thermo Scientific, Fremont, CA, U.S.A.); anti-neurofilament protein (NF) for nerve fibers (DAKO); anti-neuron specific enolase (NSE) for nerve cells (DAKO); anti-alpha smooth muscle actin (α-SMA) for myoepithelial cells (DAKO); and anti-activated cysteine aspartic acid specific protease (cleaved caspase-3) for apoptotic cells (Cell Signaling Technology, Inc., Beverley, MA, U.S.A.). Briefly, tissue sections were treated for the activation of antigens with 0.25% trypsin at RT for 30 min for anti-P antibodies, microwaving at 750W for 5 min for anti CD-3, CD20 and CD79α antibodies, heating in a water bath at 95°C for 15 min for anti-cleaved caspase-3 antibodies, proteinase K for 15 min for anti-laminin antibodies or 30 min for anti-NF antibodies, and autoclaving at 121°C for 15 min for anti-NSE and anti-α-SMA antibodies. To remove endogenous peroxidase, sections were treated with 0.3% or 3% H2O2 in methanol. To block nonspecific reactions, sections were treated with 10% normal goat serum. Primary antibodies were diluted 1:1,000 (anti-P and anti-α-SMA), 1:100 (anti-laminin, anti-NSE and anti-NF) or 1:50 (anti-cleaved caspase-3 and anti-CD3) in PBS and incubated at RT for 30 min to 2 hr or at 4°C overnight in a humidified chamber. Anti-rabbit IgG (Nichirei, Tokyo, Japan) was used as a secondary antibody for anti-P. The Envision + System Labeled Polymer-HRP anti-rabbit antibody (DAKO) was used for detection of CD3, CD20, CD79α, laminin, cleaved caspase-3 and NSE. The Histofine Simple Stain MAX-PO anti-mouse antibody was used for detection of α-SMA and NF. Antibodies were visualized using 3–3′-diaminobenzidine (DAB; DAKO). Slides were counterstained with hematoxylin.
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay: The presence of fragmented DNA was evaluated using TUNEL assays as described previously [19]. Briefly, after deparaffinizing the sections, endogenous peroxidase activity was removed with 0.3% H2O2 in methanol for 30 min at RT. Sections were then treated with 20 mg/ml proteinase-K in 0.1 M PBS for 15 min at RT to activate antigens. After washing in PBS, the slides were prepared according to the manufacturer’s guidance and were counterstained with hematoxylin.
Double immunofluorescence staining and immunostaining with a special stain: Double staining of a single tissue section was used for identification of cell type and the co-expression of antigens. After deparaffinization, sections were rehydrated in a graded alcohol series, incubated with 0.25% trypsin for 30 min to allow enzymatic-induced antigen retrieval and incubated with 10% normal goat serum to block the non-specific binding. Sections were incubated with a mixture of the primary antibodies, rabbit anti-P together with anti-α-SMA overnight at 4°C. Dilution for anti-P and anti- α-SMA antibodies was performed as described above. After washing, sections were incubated with a mixture of fluorochrome-conjugated regents, Alexa Fluor 546-goat anti-rabbit IgG (Molecular Probes, Eugene, OR, U.S.A.) together with 488-goat anti-mouse IgG (Molecular Probes), for 30 min at RT, rinsed in PBS and mounted with ProLong® Gold Antifade Mountant with DAPI (Molecular Probes). For immunostaining combination with special stain, anti-P antibody staining was performed as described above, and slides were incubated with DAB until color developed. The reaction was stopped by washing the slides in distilled water, and the slides were then incubated in 3% acetic acid for 5 min, followed by staining with Alcian blue solution for 30 min. Slides were then washed in distilled water for 5 min, stained with Nuclear-fast red for 1 min, washed in distilled water for 5 min and mounted for light microscopy.
RESULTS
Clinical characteristics of the dogs: Eighteen dogs were discovered after having died, while four dogs were subjected to euthanasia. The 22 dogs (11 males, 8 females and 3 of unknown sex) ranged in age from 1 to more than 24 months, with six dogs having unknown ages. Sixteen of the 22 dogs had no history of rabies vaccination, one dog had a history of rabies vaccination, and no information on rabies vaccination status was available for five dogs. Sixteen dogs were free-roaming dogs with owners, three were strays, two were confined with household contact, and one had unknown living conditions. The primary clinical symptoms of canine rabies infection, such as unprovoked aggressiveness, mad biting of inanimate objects, aimless running and excessive salivation, were observed in 18 of the 22 dogs. All brain specimens were diagnosed as positive for rabies virus antigen through the direct immunofluorescence antibody test (dFAT). The clinical information and results of dFAT are summarized in Table 1.
Table 1. Clinical information and laboratory findings of rabid dogs (samples obtained postmortem).
| No. | Age (months) | Sex | Owned | Manner of death | Vaccination | Animal conditions | Neurological symptoms | dFAT |
|---|---|---|---|---|---|---|---|---|
| 1 | 48 | Male | Yes | Found dead | No | Free-roaming and owned | Unprovoked aggressiveness, excessive salivation | + |
| 2 | Unknown | Male | Yes | Euthanasia | Unknown | Stray | Unprovoked aggressiveness, mad biting of inanimate objects | + |
| 3 | 24 | Female | Yes | Found dead | Yes | Free-roaming and owned | Excessive salivation, mad biting of inanimate objects, apprehensive, watchful look, paralysis | + |
| 4 | 1 | Male | Yes | Found dead | No | Free-roaming and owned | Unknown | + |
| 5 | 6 | Male | Yes | Found dead | No | Free-roaming and owned | Unprovoked aggressiveness, mad biting of inanimate objects | + |
| 6 | 6 | Male | Yes | Found dead | No | Free-roaming and owned | Unknown | + |
| 7 | 1 | Male | Yes | Found dead | No | Free-roaming and owned | Unprovoked aggressiveness | + |
| 8 | 60 | Male | Yes | Found dead | No | Confined with household contact | Unprovoked aggressiveness, excessive salivation, apprehension, watchful look, mad biting of inanimate objects | + |
| 9 | 2 | Female | Yes | Found dead | No | Free-roaming and owned | Unprovoked aggressiveness, mad biting of inanimate objects, aimless running | + |
| 10 | 12 | Female | Yes | Found dead | No | Free-roaming and owned | Unknown | + |
| 11 | 1 | Female | Yes | Found dead | No | Unknown | Unknown | + |
| 12 | 36 | Male | Yes | Found dead | No | Free-roaming and owned | Unprovoked aggressiveness, mad biting of inanimate objects, paralysis of jaw and tongue | + |
| 13 | 16 | Female | Unknown | Found dead | No | Free-roaming and owned | Unprovoked aggressiveness | + |
| 14 | Unknown | Unknown | Unknown | Euthanasia | Unknown | Stray | Unprovoked aggressiveness | + |
| 15 | 2 | Male | Unknown | Found dead | Unknown | Free-roaming and owned | Apprehensive, watchful look, paralysis | + |
| 16 | Unknown | Unknown | Unknown | Found dead | No | Free-roaming and owned | Unprovoked aggressiveness | + |
| 17 | 2 | Female | Unknown | Found dead | No | Free-roaming and owned | Unprovoked aggressiveness | + |
| 18 | 8 | Female | Unknown | Found dead | No | Free-roaming and owned | Unprovoked aggressiveness, mad biting of inanimate objects | + |
| 19 | Unknown | Male | Unknown | Euthanasia | Unknown | Free-roaming and owned | Unprovoked aggressiveness | + |
| 20 | Unknown | Male | Unknown | Found dead | No | Free-roaming and owned | Unprovoked aggressiveness, aimless running | + |
| 21 | Unknown | Unknown | Unknown | Euthanasia | Unknown | Stray | Unprovoked aggressiveness | + |
| 22 | 3 | Female | Unknown | Found dead | No | Confined with household contact | Unprovoked aggressiveness, mad biting of inanimate objects, aimless running | + |
+: Positive, dFAT: Direct fluorescent antibody test.
Histopathological findings of salivary glands of control and rabid dogs: No gross findings were observed in the parotid glands, mandibular glands and mandibular lymph nodes in control dogs (Fig. 1). The parotid gland was composed exclusively of serous acinar epithelium in association with striated ducts and interlobular excretory ducts, but interlobular ganglion cells were not found (Fig. 2). The mandibular glands were divided into several lobules by dense connective tissue septa and were composed of serous and mucous acinar epithelia. Many ganglion cells and peripheral nerve fibers were observed in the interlobular septa (Fig. 3).
Fig. 1.
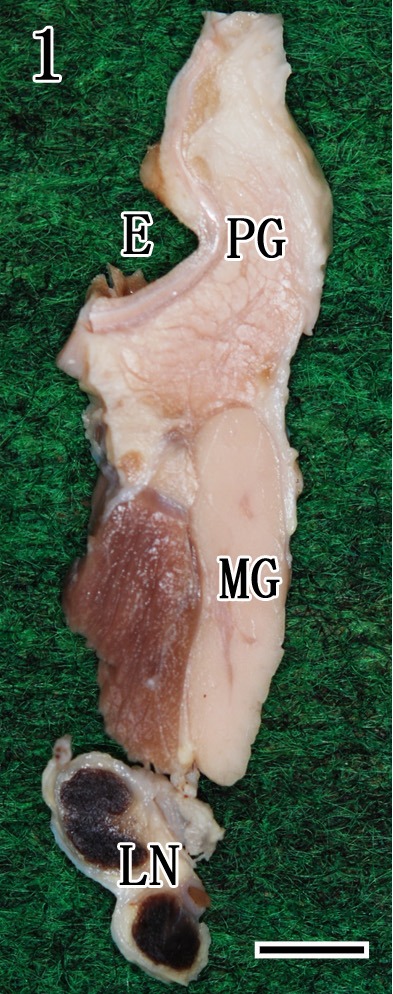
Cutting position of the right external ear (E), parotid gland (PG), mandibular gland (MG) and mandibular lymph node (LN) after formalin fixation in a control dog. Bar=1 cm.
Fig. 2.
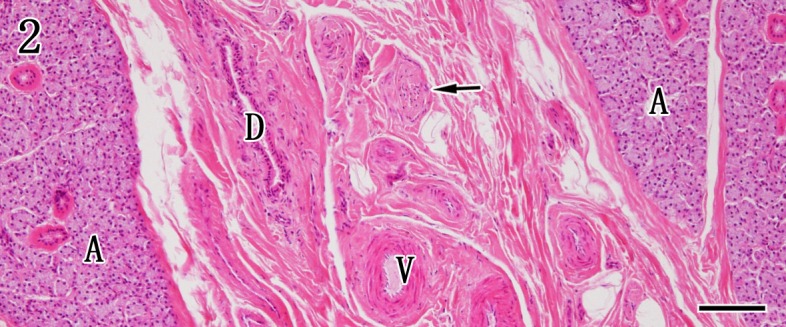
Parotid gland. The parotid gland is composed of the serous acinar gland (A) and interlobular stroma. The positions of the interlobular duct (D), peripheral nerve bundles (arrows) and vessels (V) are indicated in the interlobular stroma. HE staining. Bar=100 µm.
Fig. 3.
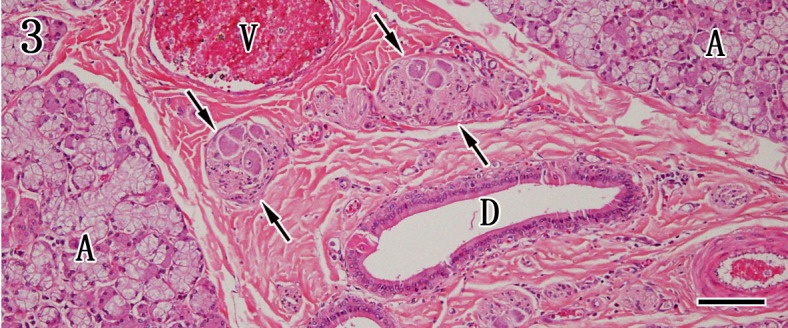
Mandibular gland. The mandibular gland is composed of mixed components of mucous and serous acinar cells (A). The positions of the interlobular duct (D), ganglion cells (arrows) and vessels (V) are indicated in the stroma. HE staining. Bar=100 µm.
In the mandibular glands, 19 out of 22 rabid dogs exhibited mild to moderate non-suppurative sialadenitis characterized by fragmentation and cytolysis of the acinar epithelium with infiltration of moderate to marked lymphocytes and plasma cells (Fig. 4). These cells consistently surrounded fragmented acini, interstitial connective tissue and striated ducts. Infiltration of inflammatory cells was not found in three of the four euthanized dogs, but small foci of necrotic acinar cells were scattered throughout the samples (Fig. 5). In all cases, no histopathological changes in striated and interlobular ductal epithelial cells and interlobular ganglion cells were found. In the parotid glands, no morphological evidence of acinar, duct units and interlobular stroma was observed (Fig. 6).
Fig. 4.
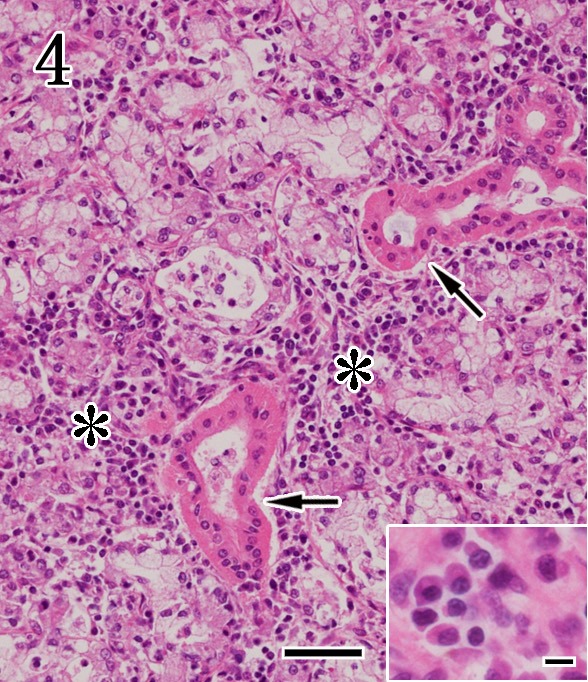
Mandibular gland. Non-suppurative sialadenitis characterized by cytolysis of acinar epithelium and infiltration of lymphoplasmacytic cells (asterisks). The striated ducts are intact (arrows). Higher magnification (inset) shows the plasma cells. HE staining. Bar=50 µm and 10 µm (inset).
Fig. 5.
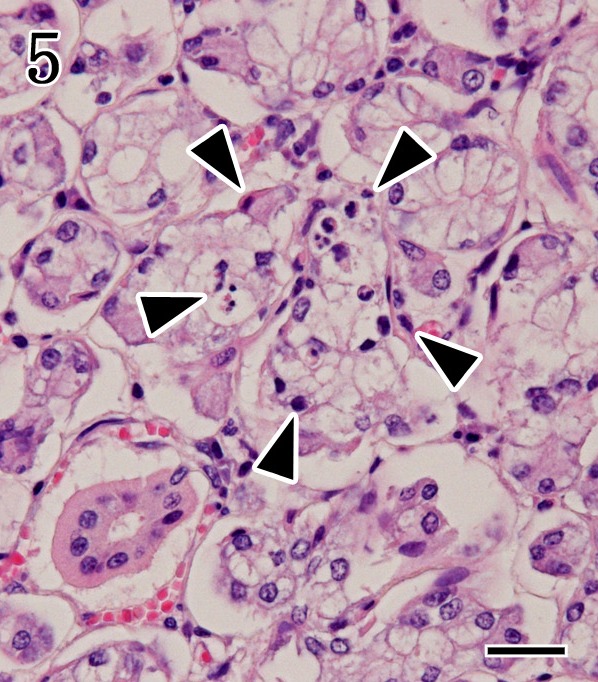
Mandibular gland. Necrotic foci of the acinar epithelium (arrowheads) without inflammatory cells were observed in the euthanasia case. HE staining. Bar=25 µm.
Fig. 6.
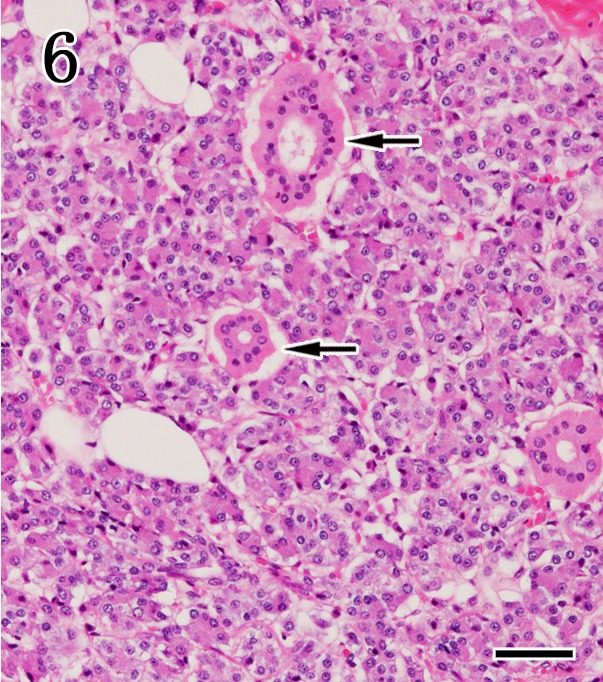
Parotid gland. No pathological findings were observed in the serous acinar epithelium or striated ducts (arrows). HE staining. Bar=50 µm.
Immunohistochemical examination of the mandibular glands and parotid glands: On immunohistochemistry using anti-rabies P antibody, no viral antigen was detected in control dogs (data not shown). In all rabid dogs, viral antigens were detected in the cytoplasm of the mucous acinar epithelium and interlobular ganglion cells in the mandibular gland (Figs. 7, 8, 9). Some viral antigens were observed in the myoepithelium and peripheral nerves, but viral antigens were not found in the striated and interlobular ductal epithelia. In parotid gland, rabies virus antigens were only detected in the interlobular peripheral nerves. These immunohistochemistry results are summarized in Table 2.
Fig. 7.
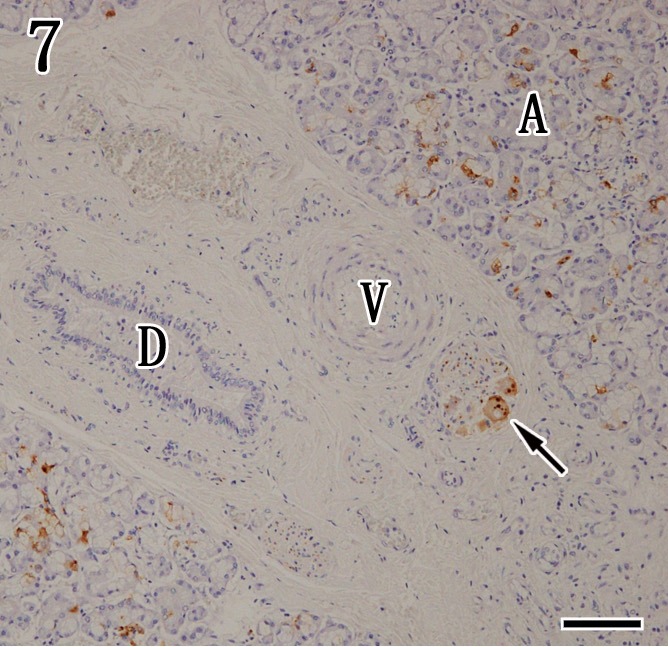
Mandibular gland. Viral antigens were detected by immunohistochemistry with anti-P antibody in the acinar epithelium (A) and ganglion cells (arrow), but the interlobular duct (D) and blood vessels (V) were negative. Immunohistochemistry. Bar=100 µm.
Fig. 8.
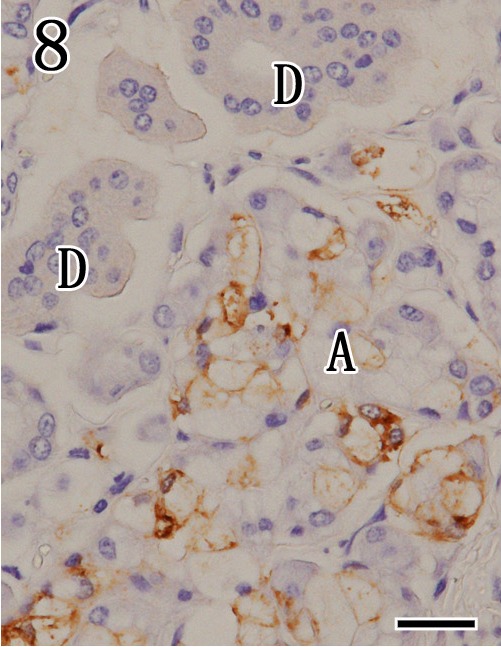
Mandibular gland. Higher magnification of Fig. 7. Viral antigens were detected by immunohistochemistry with anti-P antibodies in the cytoplasm of mucous acinar cells (A). In contrast, striated ducts (D) were negative. Immunohistochemistry. Bar=25 µm.
Fig. 9.
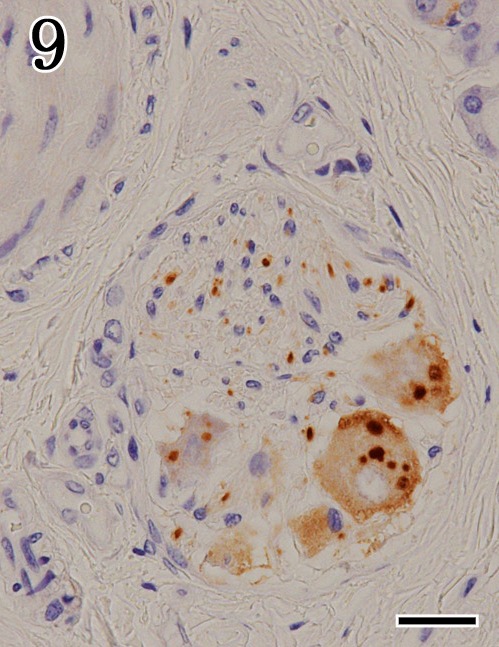
Mandibular gland. Higher magnification of Fig. 7. Viral antigens were detected by immunohistochemistry with anti-P antibodies in the ganglion cells and peripheral nerves. Immunohistochemistry. Bar=25 µm.
Table 2. Summary of inflammatory features and distribution of viral antigen in the mandibular glands of 22 rabid dogs.
| No. | Inflammatory cells | Virus antigen distribution | |||
|---|---|---|---|---|---|
| Mucous epithelium | Serous epithelium | Ductal units (striated and interlobular ducts) | Interlobular ganglion | ||
| 1 | Moderate | + | – | – | + |
| 2 | Mild | + | – | – | + |
| 3 | Mild | + | – | – | + |
| 4 | Moderate | + | – | – | + |
| 5 | Mild | + | – | – | + |
| 6 | Mild | + | – | – | + |
| 7 | Moderate | + | – | – | + |
| 8 | Moderate | + | – | – | + |
| 9 | Moderate | + | – | – | + |
| 10 | Mild | + | – | – | + |
| 11 | None | + | + | – | + |
| 12 | Mild | + | – | – | + |
| 13 | Moderate | + | – | – | + |
| 14 | None | + | – | – | + |
| 15 | Mild | + | – | – | + |
| 16 | Moderate | + | – | – | + |
| 17 | Mild | + | – | – | + |
| 18 | Moderate | + | – | – | + |
| 19 | Moderate | + | – | – | + |
| 20 | Mild | + | – | – | + |
| 21 | None | + | – | – | + |
| 22 | Moderate | + | – | – | + |
+: Positive, –: Negative.
For identification of the virus infected cells, double staining was performed by combination of anti-P antibody and either anti-α-SMA or Alcian blue staining. In the mandibular gland of rabid dogs, anti-α-SMA-positive myoepithelial cells showed co-expression with anti-P antibody reactivity (Fig. 10). In addition, the mucous acinar epithelium showed co-stained with anti-P antibody reactivity and Alcian blue staining (Fig. 11). In contrast, α-SMA-positive myoepithelial cells of the parotid glands were negative for anti-P antibody reactivity.
Fig. 10.
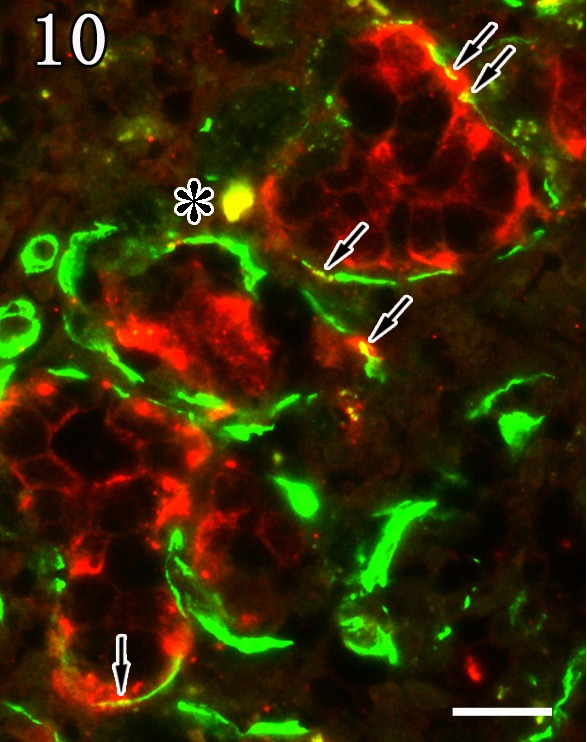
Mandibular gland. Myoepithelial cells were double positive (arrows, yellow) for anti-α-SMA (green) and anti-P antibodies (red). The asterisk indicates an artifact. Double staining. Bar=20 µm.
Fig. 11.
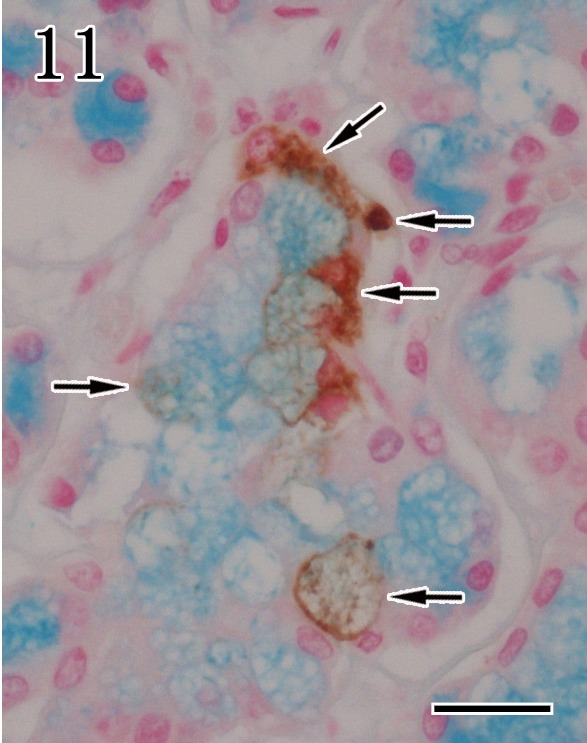
Mandibular gland. The mucous acinar epithelium showed co-expression with anti-P antibody reactivity (arrows) and Alcian blue staining. Double staining. Bar=20 µm.
Anti-α-SMA antibody was used as a myoepithelial cell marker. In control dogs, anti-α-SMA antibody was positive in the myoepithelial cells surrounding acinar epithelium (Fig. 12A) and intercalated duct epithelium of the mandibular and parotid glands. However, in rabid dogs, the intensity of immunostaining of anti-α-SMA-positive cells was decreased in glands that showed severe inflammation (Fig. 12B).
Fig. 12.
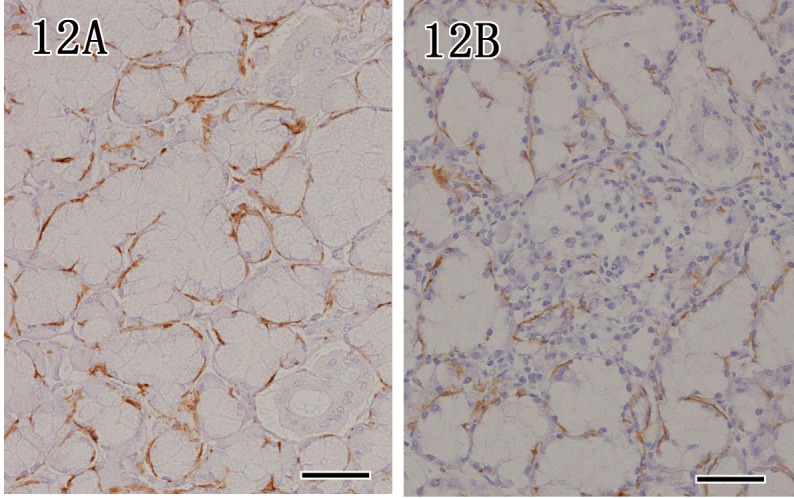
Mandibular gland. In a control dog, myoepithelial cells surrounding acinar epithelium showed strong reactivity for anti-α-SMA (12A), whereas the intensity of immunostaining decreased in rabid dogs (12B). Immunohistochemistry. Bar=50 µm.
In mandibular glands of rabid dogs, there are moderate numbers of anti-CD3 positive cells appeared in the interstitial connective tissue and around fragmented acinar epithelium, while anti-CD20 and anti-CD79α (Fig. 13) positive cells were mainly detected in the interstitial connective tissue and periductal areas. No positive cells were detected in the parotid glands.
Fig. 13.
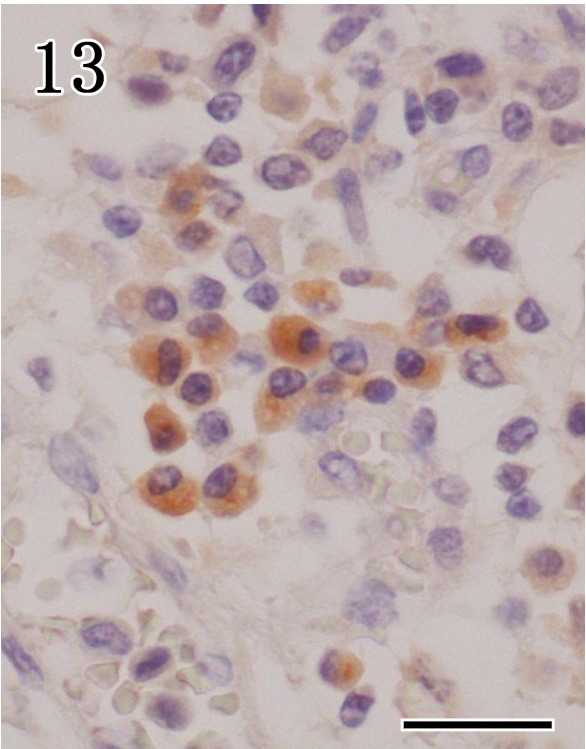
Mandibular gland. Many of anti-CD79α positive cells were observed in the interstitial connective tissue. Immunohistochemistry. Bar=20 µm.
Anti-NSE and NF antibodies were used as neuronal markers. Interlobular ganglion cells and peripheral nerve fibers were positive for anti-NSE antibodies in the mandibular glands. Interlobular ganglion cells were not found in the parotid glands. Anti-NF immunoreactivity was observed as a fine network in the nerve fibers distributed throughout the stroma and around acini in the mandibular glands. The staining patterns were the same between control and rabid dogs.
In control dogs, laminin immunoreactivity and reticulin silver impregnation staining appeared as linear, continuous staining around individual acini and ducts of the mandibular and parotid glands. In the mandibular glands of rabid dogs, staining was irregular and weak, because acinar epithelium and basement membranes were disrupted (Fig. 14).
Fig. 14.
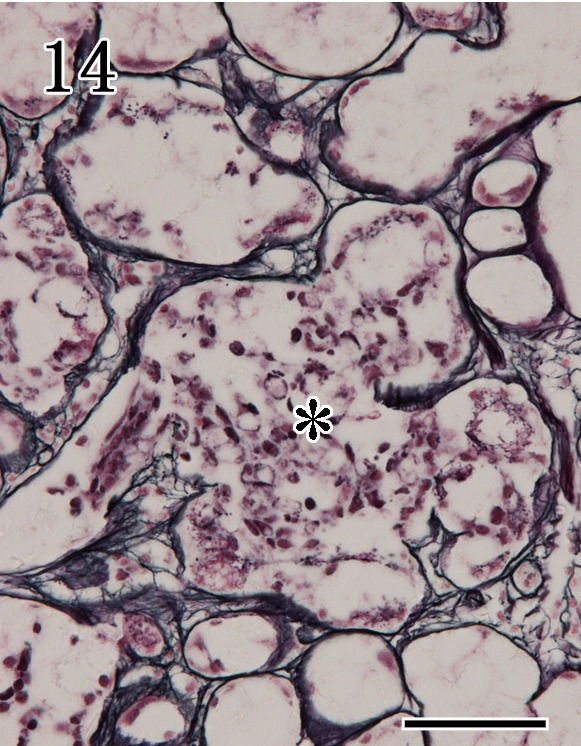
Mandibular gland. Reticulin silver impregnation staining revealed irregular positive reactions, with disruption of acinar epithelium (asterisk) and basement membranes. Special staining. Bar=50 µm.
Detection of apoptotic cells by TUNEL assays and anti-cleaved caspase-3 antibodies: In mandibular glands of rabid dogs, the acinar epithelium and lymphocytes exhibited apoptotic features, such as nuclear fragmentation and cytolysis, and were positive in TUNEL assays (Fig. 15). The numbers of TUNEL-positive cells were higher in glands that showed severe inflammation. However, TUNEL-positive signals were not detected in the ductal epithelium, interlobular ganglion cells and serous demilune. In parotid glands, the acinar epithelium and the ductal epithelium were negative for TUNEL staining. The patterns of anti-cleaved caspase-3 immunostaining were similar to those of TUNEL staining in the mandibular glands, but with fewer positive cells.
Fig. 15.
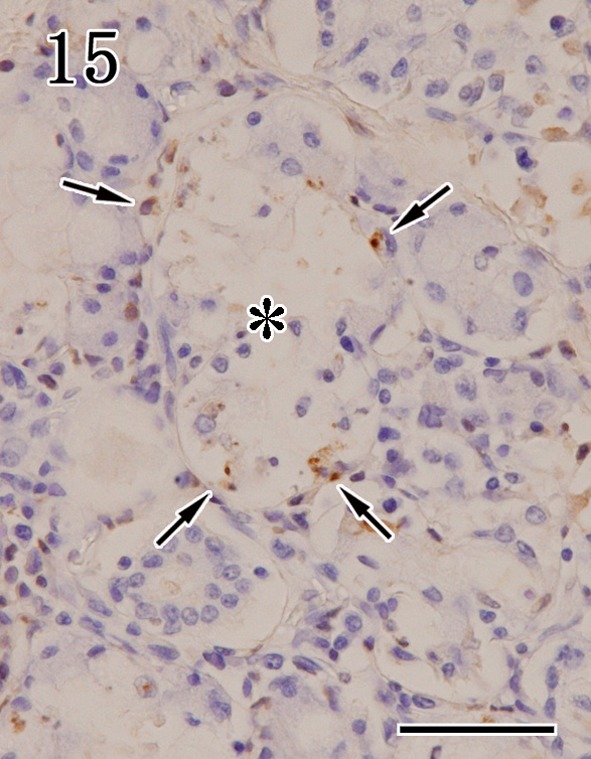
Mandibular gland. Necrotic foci (asterisk) in the acinar epithelium were TUNEL positive (arrows). TUNEL methods. Bar=50 µm.
DISCUSSION
Centrifugal spread from the CNS to peripheral sites along neuronal routes is essential for transmission of rabies virus to its natural hosts. Salivary gland infection is necessary for the transfer of infectious oral fluids by rabid vectors. In this study, non-suppurative sialadenitis, characterized by fragmentation, cytolysis of the acinar epithelium, basement membrane disruption and lymphoplasmacytic infiltration, was observed in the mandibular glands of most rabid dogs. No inflammatory cells were found in three of four euthanized dogs; however, small foci of necrotic acinar cells were scattered throughout the samples, and these cells were positive for anti-caspase-3 and TUNEL staining. These pathological findings suggested that early sialadenitis was induced by direct disruption of the acinar epithelium by rabies virus infection.
In our study, the viral antigen was mainly detected in the mucous acinar epithelium and myoepithelium in the mandibular glands. These findings are consistent with previous reports of skunks and foxes infected with the street rabies virus [1]. In addition, the viral antigen was present in the myoepithelium between the basal lamina and acinar epithelial cells. Thus, the rabies virus may propagate in the myoepithelium and affect nerve terminal innervation. Previous studies have described the morphological structure of the neuro-effector that innervates the acinar epithelium and myoepithelium of the salivary glands of carnivores [11, 20, 27]. These neuro-effectors may be the hypolemmal type (i.e., non-myelinated axons that penetrate below the acinar basement membrane and adjacent to the myoepithelium) or the epilemmal type (i.e., non-myelinated axons found outside the acinar epithelium and myoepithelium). Furthermore, the intra-acinar nerve endings in the mandibular glands are restrictively localized only in the intercellular space between myoepithelial cells and mucous acinar cells, whereas no intra-acinar nerve endings occur among the serous cells [17]. Therefore, it was suggested the rabies virus may directly enter mucous acinar epithelial cells and myoepithelial cells via both innervation. However, several mammalian species lack apparent hypolemmal innervation in the striated ducts and interlobular excretory ducts [31, 32]. In the present study, we did not observe degeneration, inflammation or viral antigen in the duct unit of striated and interlobular ducts in the mandibular gland, suggesting that hypolemmal and epilemmal innervations were not present in the duct system of dogs. Thus, viral proliferation and cytotoxicity could not occur there, ensuring that secretions containing the virus were efficiently excreted into the oral cavity.
Interlobular ganglions were observed in the interlobular septa of the mandibular glands, similar to findings in humans [34] and rats [24]. These ganglions included a number of cholinergic ganglion neurons that received motor impulses from pre- and postganglionic parasympathetic fibers carried by the facial nerve [9]. Ganglion neurons are responsible for innervation of the salivary parenchyma and for regulation of saliva secretion [24, 26]. In the present study, viral antigens were detected in the ganglion neurons and their fibers. Thus, centrifugal viral propagation progresses to the mandibular glands via motor innervations. After the rabies virus replicates within the facial nerve nuclei, located in the ventral part of the rostral medulla oblongata, the virus then descends along the facial nerve to reach the submandibular ganglion and the interlobular ganglions [3, 30, 35]. In this study, we did not observe interlobular ganglia in the parotid gland, and also no viral antigens were detected here. Therefore, it was suggested that these ganglia are important for viral replication and serve as a main source of virus to acinar epithelium of the mandibular glands.
In the present study, virus-infected ganglion neurons were negative for TUNEL staining and cleaved caspase-3, indicating that virus-infected neurons did not undergo apoptosis, similar to results reported in natural infection of dogs and humans by a street rabies virus strain [15, 30]. Therefore, we concluded that lack of neuronal apoptosis in rabies in dogs infected by street virus may promote prolonged infection within ganglion neurons and continually supply virus to the salivary acinar epithelium. On the other hand, virus-infected mucous acinar epithelial cells exhibited fragmentation and cytolysis, and migrating T lymphocytes were positive for TUNEL and cleaved caspase-3, suggesting that rabies virus may trigger apoptotic cascades through the Fas/Fas ligand and caspase-dependent apoptotic pathways [29].
In the present study, the inflammatory cells were composed mainly of CD3, CD20 and CD79α-positive lymphocytes and were present in the virus-infected mandibular gland. These findings suggested that a combination of cell-mediated and humoral immune responses was important for the clearance of rabies virus from the salivary gland and that the inflammatory cytokines released by T lymphocytes activated plasma cell infiltration. CD79α is a surface marker of B lymphocytes and plasma cells. B lymphocytes play an important role in producing virus-neutralizing antibodies and are critical for control of virus replication and essential in the clearance rabies virus from the CNS [13]. In addition, high titers of tissue-neutralizing antibody suppress viral spread to salivary epithelial cells [4]. The plasma cells in the salivary tissue are the source of locally produced antibodies, such as immunoglobulin A (IgA), which provides mucosal surface immunity against various antigens and neutralizes viruses [5, 22]. Furthermore, IgA-producing plasma cells infiltrate the salivary gland via regulation of T lymphocytes and various inflammatory cytokines, such as interleukin (IL)-5 and interferon (IFN)-α [23]. In the present study, however, the direct roles of lymphocytes in the salivary gland of rabid dogs remain unresolved, because most of present cases were discovered after died, and therefore, further studies are required.
In summary, our results confirmed the path through which the rabies virus descends along the facial nerve after proliferation in the brain to reach the ganglion neurons of the mandibular gland, subsequently traveling to the acinar epithelium and salivary gland myoepithelium. Furthermore, viral proliferation and cytotoxicity did not occur in the duct system, ensuring that secretions containing the virus were efficiently excreted into the oral cavity.
Acknowledgments
The authors would like to acknowledge the invaluable help of staff of the Research Institute for Tropical Medicine (RITM), Department of Health, Philippines for tissue collection of dogs and the permission to use these sample for the current study. This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Kakenhi No. 26450410) and was partly supported by a grant for scientific research from the KITASATO University and Heiwa Nakajima Foundation, Japan.
REFERENCES
- 1.Balachandran A., Charlton K.1994. Experimental rabies infection of non-nervous tissues in skunks (Mephitis mephitis) and foxes (Vulpes vulpes). Vet. Pathol. 31: 93–102. doi: 10.1177/030098589403100112 [DOI] [PubMed] [Google Scholar]
- 2.Banyard A. C., Horton D. L., Freuling C., Müller T., Fooks A. R.2013. Control and prevention of canine rabies: the need for building laboratory-based surveillance capacity. Antiviral Res. 98: 357–364. doi: 10.1016/j.antiviral.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 3.Charlton K. M., Casey G. A., Campbell J. B.1983. Experimental rabies in skunks: mechanism of infection of the salivary glands. Can. J. Comp. Med. 47: 363–369. [PMC free article] [PubMed] [Google Scholar]
- 4.Charlton K. M., Casey G. A., Campbell J. B.1987. Experimental rabies in skunks: immune response and salivary gland infection. Comp. Immunol. Microbiol. Infect. Dis. 10: 227–235. doi: 10.1016/0147-9571(87)90033-6 [DOI] [PubMed] [Google Scholar]
- 5.Corthésy B.2010. Role of secretory immunoglobulin A and secretory component in the protection of mucosal surface. Future Microbiol. 5: 817–829. doi: 10.2217/fmb.10.39 [DOI] [PubMed] [Google Scholar]
- 6.Dierks R. E., Murphy F. A., Harrison A. K.1969. Extraneural rabies virus infection: virus development in fox salivary gland. Am. J. Pathol. 54: 251–273. [PMC free article] [PubMed] [Google Scholar]
- 7.Dietzschold B., Li J., Faber M., Schnell M.2008. Concepts in the pathogenesis of rabies. Future Virol. 3: 481–490. doi: 10.2217/17460794.3.5.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimaano E. M., Scholand S. J., Alera M. P., Belandres D. B.2011. Clinical and epidemiological features of human rabies cases in the Philippines: a review from 1987 to 2006. Int. J. Infect. Dis. 15: e495–499. doi: 10.1016/j.ijid.2011.03.023 [DOI] [PubMed] [Google Scholar]
- 9.Evans H. E.1993. pp. 953–986. In: Miller’s Anatomy of the Dog, 3rd ed., W. B. Saunders Company, Philadelphia. [Google Scholar]
- 10.Fekadu M., Shaddock J. H.1984. Peripheral distribution of virus in dogs inoculated with two strains of rabies virus. Am. J. Vet. Res. 45: 724–729. [PubMed] [Google Scholar]
- 11.Garrett J. R., Kidd A.1993. The innervation of salivary glands as revealed by morphological methods. Microsc. Res. Tech. 26: 75–91. doi: 10.1002/jemt.1070260108 [DOI] [PubMed] [Google Scholar]
- 12.Hemachudha T., Laothamatas J., Rupprecht C. E.2002. Human rabies: a disease of complex neuropathogenic mechanisms and diagnostic challenge. Lancet Neurol. 1: 101–109. doi: 10.1016/S1474-4422(02)00041-8 [DOI] [PubMed] [Google Scholar]
- 13.Hooper D. C., Phares T. W., Fabris M. J., Roy A.2009. The production of antibody by invading B cells is required for the clearance of rabies virus from the central nervous system. PLoS Negl. Trop. Dis. 3: e535. doi: 10.1371/journal.pntd.0000535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson A. C., Wunner W. H.2007. pp. 341–372. In: Rabies, 2nd ed., Elsevier Saunders, Philadelphia. [Google Scholar]
- 15.Jackson A. C., Randle E., Lawrance G., Rossiter J. P.2008. Neuronal apoptosis does not play an important role in human rabies encephalitis. J. Neurovirol. 14: 368–375. doi: 10.1080/13550280802216502 [DOI] [PubMed] [Google Scholar]
- 16.Jogai S., Radotra B. D., Banerjee A. K.2002. Rabies viral antigen in extracranial organs: a post-mortem study. Neuropathol. Appl. Neurobiol. 28: 334–338. doi: 10.1046/j.1365-2990.2002.00400.x [DOI] [PubMed] [Google Scholar]
- 17.Kagayama M.1971. The fine structure of the monkey submandibular gland with a special reference to intra-acinar nerve endings. Am. J. Anat. 131: 185–195. doi: 10.1002/aja.1001310204 [DOI] [PubMed] [Google Scholar]
- 18.Kasempimolporn S., Saengseesom W., Lumlertdacha B., Sitprija V.2000. Detection of rabies virus antigen in dog saliva using a latex agglutination test. J. Clin. Microbiol. 38: 3098–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kojima D., Park C. H., Satoh Y., Inoue S., Noguchi A., Oyamada T.2009. Pathology of the spinal cord of C57BL/6J mice infected with rabies virus (CVS-11). J. Vet. Med. Sci. 71: 319–324. doi: 10.1292/jvms.71.319 [DOI] [PubMed] [Google Scholar]
- 20.König Júnior B., Masuko T. S.1998. Ultrastructure of the parotid and submandibular glands of the Old World Marten (Carnivora; Mustelidae). Ann. Anat. 180: 31–36. doi: 10.1016/S0940-9602(98)80127-1 [DOI] [PubMed] [Google Scholar]
- 21.Lewis P., Fu Y., Lentz T. L.2000. Rabies virus entry at the neuromuscular junction in nerve-muscle cocultures. Muscle Nerve 23: 720–730. doi: [DOI] [PubMed] [Google Scholar]
- 22.Mestecky J.1993. Saliva as a manifestation of the common mucosal immune system. Ann. N. Y. Acad. Sci. 694: 184–194. doi: 10.1111/j.1749-6632.1993.tb18352.x [DOI] [PubMed] [Google Scholar]
- 23.Mega J., McGhee J. R., Kiyono H.1992. Cytokine- and Ig-producing T cells in mucosal effector tissues: analysis of IL-5- and IFN-gamma-producing T cells, T cell receptor expression, IgA plasma cells from mouse salivary gland-associated tissue. J. Immunol. 148: 2030–2039. [PubMed] [Google Scholar]
- 24.Ng Y. K., Wong W. C., Ling E. A.1992. The intraglandular submandibular ganglion of postnatal and adult rats I. A light and electron microscopic study. J. Anat. 180: 305–314. [PMC free article] [PubMed] [Google Scholar]
- 25.Prager K. C., Mazet J. A., Dubovi E. J., Frank L. G., Munson L., Wagner A. P., Woodroffe R.2012. Rabies virus and canine distemper virus in wild and domestic canivores in northen Kenya: are domestic dogs the reservoir? EcoHealth 9: 483–498. doi: 10.1007/s10393-013-0815-9 [DOI] [PubMed] [Google Scholar]
- 26.Proctor G. B., Carpenter G. H.2007. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 133: 3–18. doi: 10.1016/j.autneu.2006.10.006 [DOI] [PubMed] [Google Scholar]
- 27.Redman R. S.1994. Myoepithelium of salivary glands. Microsc. Res. Tech. 27: 25–45. doi: 10.1002/jemt.1070270103 [DOI] [PubMed] [Google Scholar]
- 28.Saengseesom W., Mitmoonpitak C., Kasempimolporn S., Sitprija V.2007. Real-Time PCR analysis of dog cerebrospinal fluid and saliva samples for ante-mortem diagnosis of rabies. Southeast Asian J. Trop. Med. Public Health 38: 53–57. [PubMed] [Google Scholar]
- 29.Sarmento L., Tseggai T., Dhingra V., Fu Z. F.2006. Rabies virus-induced apoptosis involves caspase-dependent and caspase-independent pathways. Virus Res. 121: 144–151. doi: 10.1016/j.virusres.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 30.Suja M. S., Mahadevan A., Madhusudana S. N., Vijayasarathi S. K., Shankar S. K.2009. Neuroanatomical mapping of rabies nucleocapsid viral antigen distribution and apoptosis in pathogenesis in street dog rabies: an immunohistochemical study. Clin. Neuropathol. 28: 113–124. doi: 10.5414/NPP28113 [DOI] [PubMed] [Google Scholar]
- 31.Tandler B., Gresik E. W., Nagato T., Phillips C. J.2001. Secretion by striated ducts of mammalian major salivary glands: review from an ultrastructural, functional, and evolutionary perspective. Anat. Rec. 264: 121–145. doi: 10.1002/ar.1108 [DOI] [PubMed] [Google Scholar]
- 32.Tandler B., Pinkstaff C. A., Phillips C. J.2006. Interlobular excretory ducts of mammalian salivary glands: structural and histochemical review. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 288: 498–526. doi: 10.1002/ar.a.20319 [DOI] [PubMed] [Google Scholar]
- 33.Tobiume M., Sato Y., Katano H., Nakajima N., Tanaka K., Noguchi A., Inoue S., Hasegawa H., Iwasa Y., Tanaka J., Hayashi H., Yoshida S., Kurane I., Sata T.2009. Rabies virus dissemination in neural tissues of autopsy cases due to rabies imported into Japan from the Philippines: Immunohistochemistry. Pathol. Int. 59: 555–566. doi: 10.1111/j.1440-1827.2009.02406.x [DOI] [PubMed] [Google Scholar]
- 34.Tosios K. I., Nikolakis M., Prigkos A. C., Diamanti S., Sklavouvou A.2010. Nerve cell bodies and small ganglia in the connective tissue stroma of human submandibular glands. Neurosci. Lett. 475: 53–55. doi: 10.1016/j.neulet.2010.03.045 [DOI] [PubMed] [Google Scholar]
- 35.Umoh J. U., Blenden D. C.1982. The dissemination of rabies virus into cranial nerves and other tissues of experimentally infected goats and dogs and naturally infected skunks. Int. J. Zoonoses 9: 1–11. [PubMed] [Google Scholar]
- 36.WHO2013. Expert consultation on rabies. Second report. World Health Organ. Tech. Rep. Ser. 982: 1–139. [PubMed] [Google Scholar]


