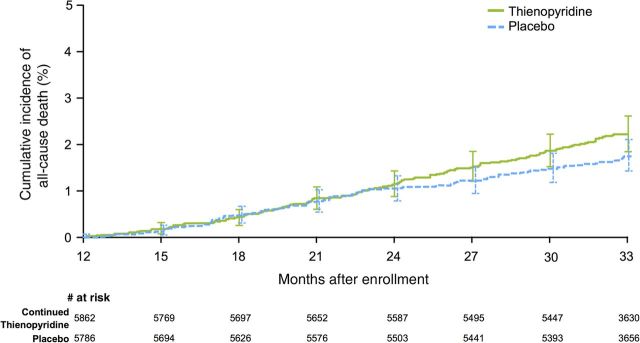
Figure 1.
Cumulative incidence curves for all-cause mortality. Randomization occurred at 12 months after stenting. The primary analysis period was the period from Months 12 to 30 after percutaneous coronary intervention, during which subjects received the randomized study drug. Patients were followed for an observational period of an additional 3 months after discontinuation of the study drug (i.e. to 33 months after enrolment). From 12 to 30 months, 1.9% in the thienopyridine arm died compared with 1.5% in the placebo arm (hazard ratio 1.31, P = 0.07). From 12 to 33 months, 2.2 vs. 1.8% mortality was observed in the thienopyridine and placebo arms, respectively (hazard ratio 1.32, P = 0.05).