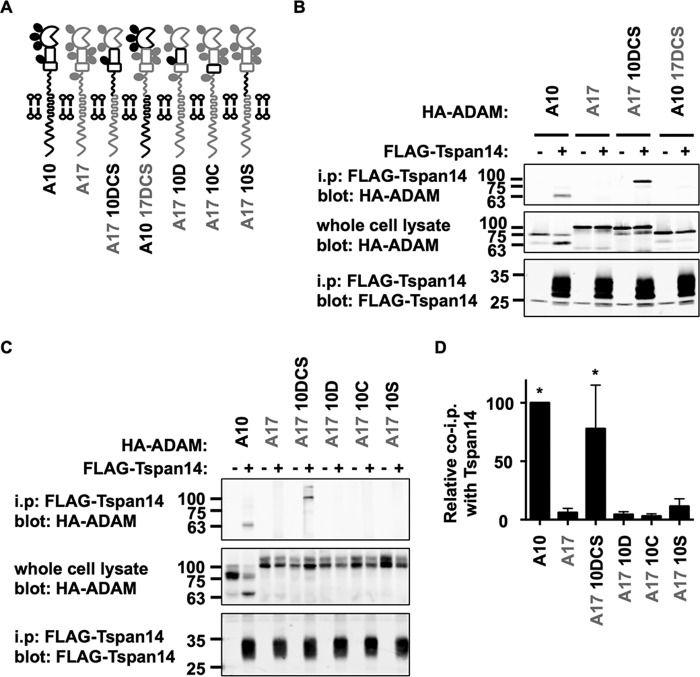
FIGURE 5.
The region of ADAM10 comprising the disintegrin domain (D), the cysteine-rich (C), and stalk (S) regions mediates the interaction with Tspan14. A, schematic of ADAM10 and ADAM17 chimeras. The extracellular disintegrin (D), cysteine-rich (C), and stalk (S) regions of ADAM10 (black) and ADAM17 (gray) were interchanged together (DCS) or individually. B, HEK-293T cells were mock transfected (−) or transfected with FLAG-tagged mouse Tspan14 (+) in addition to either HA-tagged mouse ADAM10, ADAM17, ADAM17 10DCS, or ADAM10 17DCS. Cells were lysed in 1% digitonin lysis buffer and immunoprecipitated with an anti-FLAG antibody. Immunoprecipitated proteins were blotted with anti-HA tag antibody (top panel) or anti-FLAG antibody (lower panel). Whole cell lysates were probed with the anti-HA tag antibody (middle panel). The blots are representative of three independent experiments. C, HEK-293T cells were co-transfected with (+) or without (−) FLAG-tagged mouse Tspan14 and either HA-mouse ADAM10, ADAM17, ADAM17 10DCS, ADAM17 10D, ADAM17 10C, or ADAM17 10S. Cells were treated as in B. D, data from panels B and C were quantitated and presented as the relative amount of each ADAM10/17 construct immunoprecipitated with Tspan14, having arbitrarily set wild-type ADAM10 to 100. Data were normalized by log transformation and statistically analyzed using a one-way ANOVA with a Dunnett's multiple comparison test, compared with the ADAM17 control (*, p < 0.05). Error bars represent standard errors of the mean from 3–6 experiments.