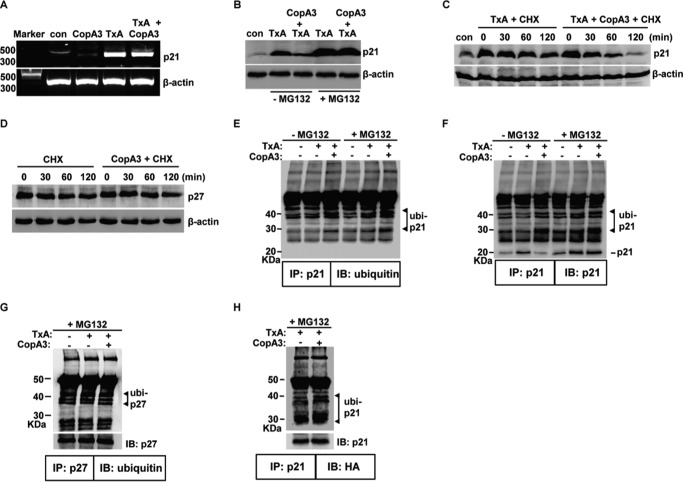
FIGURE 7.
The CopA3-dependent enhancement of ubiquitination shortens the protein half-life of p21Cip1/Waf1. A, HT29 cells (105 cells/well) were pretreated with CopA3 (20 μg/ml) for 1 h and then incubated with medium (con), CopA3 alone, toxin A (TxA, 3 nm) alone, or toxin A plus CopA3 for 6 h. Total RNA was isolated, cDNA was synthesized, and p21Cip1/Waf1 and β-actin were amplified by PCR. The results shown are representative of three separate experiments. B, cells were pretreated with CopA3 for 1 h in the presence or absence of MG132 (10 μm) and then incubated with medium (con), toxin A (TxA) alone, or toxin A plus CopA3 for 24 h. Cell lysates were resolved by 15% SDS-PAGE, and the blots were probed with antibodies against p21Cip1/Waf1 and β-actin. C, cells were treated with toxin A for 6 h (to increase the levels of p21Cip1/Waf1) and then incubated with cycloheximide alone (CHX, 100 μm) or cycloheximide plus CopA3 for the indicate times. The results shown are representative of three separate experiments. D, the protein half-life of p27Kip1 was assessed in cells exposed to CopA3. E and F, cells were pretreated with CopA3 for 1 h in the presence or absence of MG132 and further incubated with medium, toxin A alone, or toxin A plus CopA3. After 24 h, cell extracts were immunoprecipitated (IP) with an anti-p21Cip1/Waf1 antibody (p21) and resolved by 10% SDS-PAGE, and blots were probed (IB) with antibodies against ubiquitin or p21Cip1/Waf1. G, the ubiquitination levels of p27Kip1 (p27) were assessed in cells exposed to CopA3. H, HT29 cells were transiently transfected with a vector expressing HA-tagged ubiquitin and incubated with MG132 and toxin A alone or toxin A plus CopA3. After 24 h, cell extracts were IP with an anti-p21Cip1/Waf1 antibody, the immunoprecipitates were resolved by 10% SDS-PAGE, and the blots were probed with antibodies against HA (for ubiquitinated p21Cip1/Waf1) and p21Cip1/Waf1.