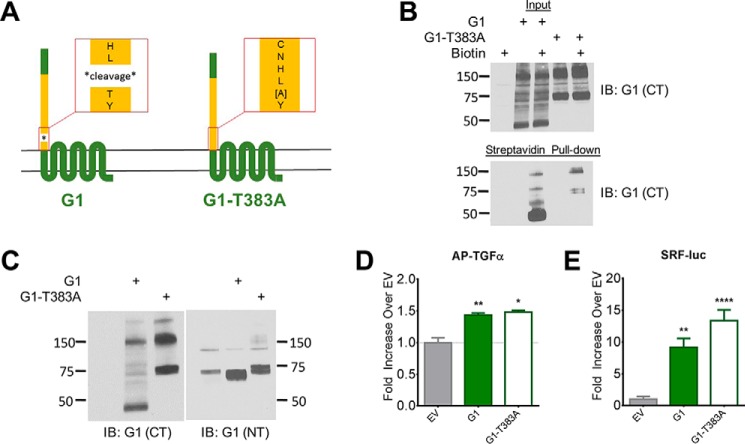
FIGURE 2.
GAIN domain cleavage is not necessary for G1 activity. A, schematic of T383A point mutation in G1. B, G1-T383A is expressed on HEK cell surface, albeit at a reduced level compared with the wild-type receptor. Molecular weight markers (in kDa) are shown on the left side of the blots. C, Western blots of G1 and G1-T383A reveal a ∼75 kDa band for G1-T383A that is both N-terminally and C-terminally reactive, suggesting non-cleavage of the mutant receptor. Equal amounts of protein (10–20 μg) were loaded in each lane for the blots shown in panels B and C, and these experiments were performed 3–4 times each. D and E, G1 and G1-T383A produce comparable activity in the AP-TGFα shedding and SRF-luciferase assays. Results for TGFα and SRF-luc are from 3–6 independent experiments (± S.E. shown, *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus cells transfected with empty vector, denoted by EV).