FIGURE 2.
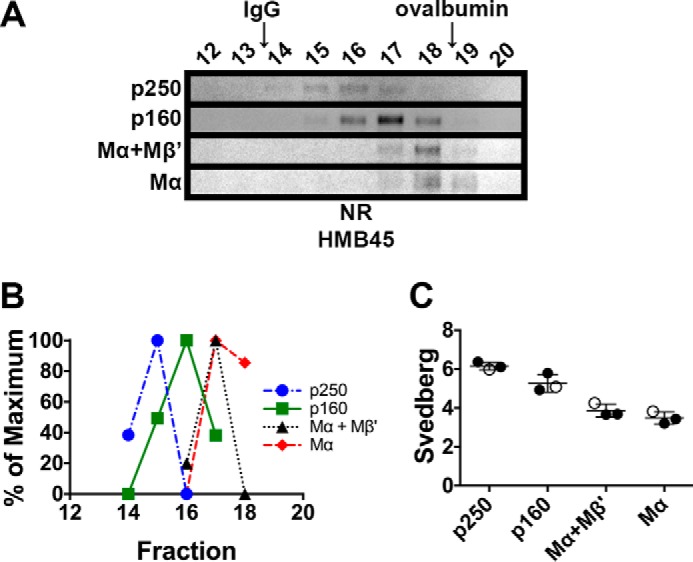
p250, p160, and Mα monomers have distinct sedimentation properties. MNT-1 cell lysates prepared using 250 mm n-octylglucoside were subject to sedimentation velocity analyses in 5–20% sucrose gradients. A, aliquots of the gradient fractions were analyzed by non-reducing (NR) SDS-PAGE and immunoblotted using HMB45. Shown are regions of the immunoblots corresponding to p250, p160, Mα+Mβ′, and Mα, as indicated to the left. Fraction numbers are indicated above each lane (bottom of the gradient is to the left, and top of the gradient is to the right), and the migration of the globular protein standards, IgG and ovalbumin, are indicated with arrows at the top. Note that immunoblot contrast was optimally adjusted for each individual species. B, HMB45 immunoreactivity was quantified to determine the peak elution fraction for p250, p160, Mα+Mβ′, and Mα. The lowest value quantified for each species was set to 0%, and the highest value was set to 100%. C, Svedberg values were calculated for each species by comparing peak fractions with those of protein standards with known s values in three independent experiments. Circles, values calculated from each experiment; open circles, values calculated from the experiment shown in A and B; horizontal lines, mean value; error bars, S.D. Mean ± S.D. are also shown in Table 1.
