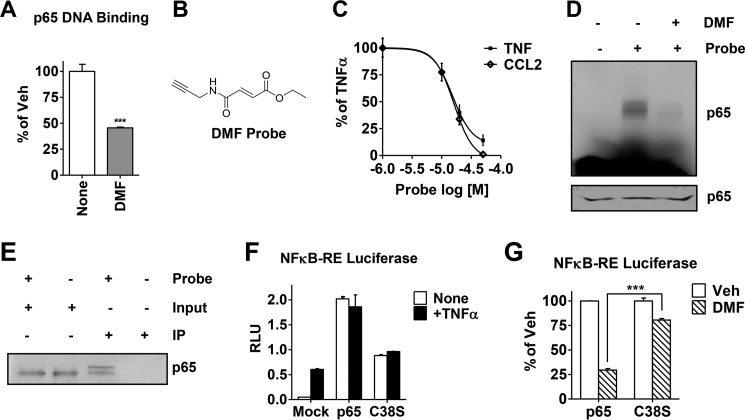
FIGURE 6.
DMF covalently modifies p65 both in vitro and in cell lysates, and cysteine 38 is the key residue responsible for DMF activity on the NFκB pathway. A, recombinant p65 DNA binding activity was measured after incubation with DMF (50 μm) for 30 min. Data are plotted as percent vehicle (Veh) control. ***, p < 0.001. B, the chemical structure of the novel alkyne-based DMF probe. C, expression of the NFκB target genes TNF and CCL2 in MCF-7 cells was measured by quantitative RT-PCR upon treatment with varying concentrations of the DMF probe, followed by TNFα. Data are plotted as percent of TNFα alone. D, gel image for labeling of recombinant p65 with the DMF probe (50 μm) measured by in-gel fluorescence. Third lane, DMF (50 μm) was added for 30 min prior to incubation with the DMF probe. Bottom panel, Coomassie staining of the gel indicating equal protein loading. E, biotin IP was carried out in MDA-MB-231 cell lysates cross-linked in the presence or absence of the DMF probe (50 μm). Total p65 protein was then immunoblotted and compared between IPs and inputs (10% of the protein lysate load). F, NFκB-RE activity (shown in relative luciferase units, RLU) was measured in MCF-7 cells transfected with wild-type p65 or C38S-p65 or mock-transfected and then treated with or without TNFα for 4 h. G, NFκB-RE activity was measured in MCF-7 cells transfected with wild-type p65 or C38S-p65 and treated with DMF (50 μm). Data are presented as percent inhibition relative to the vehicle (Veh) control. ***, p < 0.001.