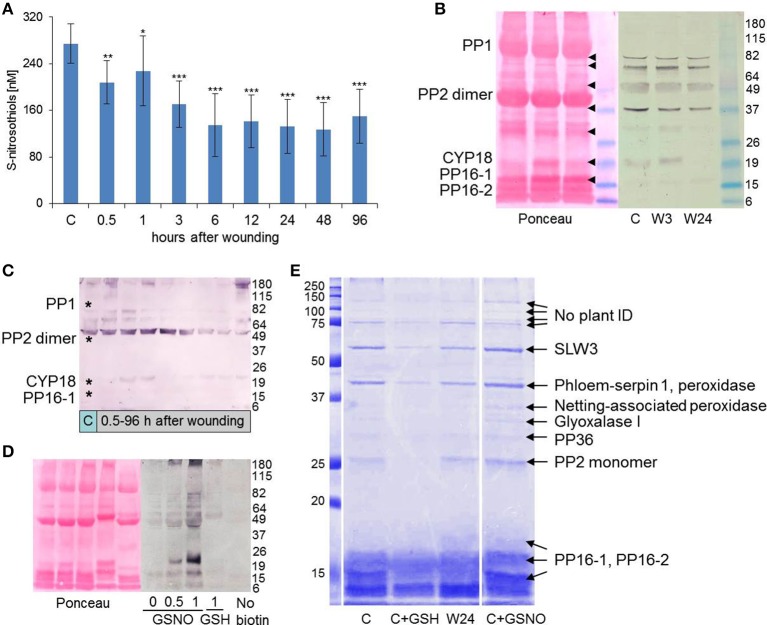
Figure 3.
Cysteine S-nitrosylation is reduced in the EFP after leaf wounding. (A) Total S-nitrosothiols were determined using a Sievers Nitric Oxide Analyzer. Columns represent means (±SD, n = 7–11). Asterisks indicate significant differences from control (C) samples (Student's t-test, *p < 0.05, **p < 0.01, ***p < 0.005). (B–D) Anti-biotin western blot (WB) analyses for detection of S-nitrosylated EFP proteins after biotinylation of S-nitrosylated proteins by the biotin switch assay. Successful protein transfer was confirmed by Ponceau Red staining of the nitrocellulose membrane. Molecular weight (kD) of protein standards are indicated on the right. (B) Comparison of protein S-nitrosylation between phloem sap from untreated control pumpkin plants (C) and wounded plants (W3, W24, 3, and 24 h after wounding). Major phloem proteins are labeled (cf. Walz et al., 2004). Note that the non-reducing buffers used for the WB facilitated the formation of PP2 dimers (cf. Read and Northcote, 1983). Arrow heads indicate positions of WB signals on the Ponceau Red stained membrane. (C) Time course of protein S-nitrosylation after wounding. Asterisks mark the positions of major phloem proteins. (D) Effect of 0.5 and 1 mM GSNO and 1 mM GSH on S-nitrosylation. One non-biotinylated control is shown. (E) Isolation, separation and identification of S-nitrosylated EFP proteins. Proteins identified in cut bands are indicated. Phloem sap from control plants (C) was treated or not with 8 mM GSH or 8 mM GSNO for maximal protein yield. Note that S-nitrosylation is decreased in phloem sap from a wounded plant (W24). Protein standards are labeled on the left (kD).