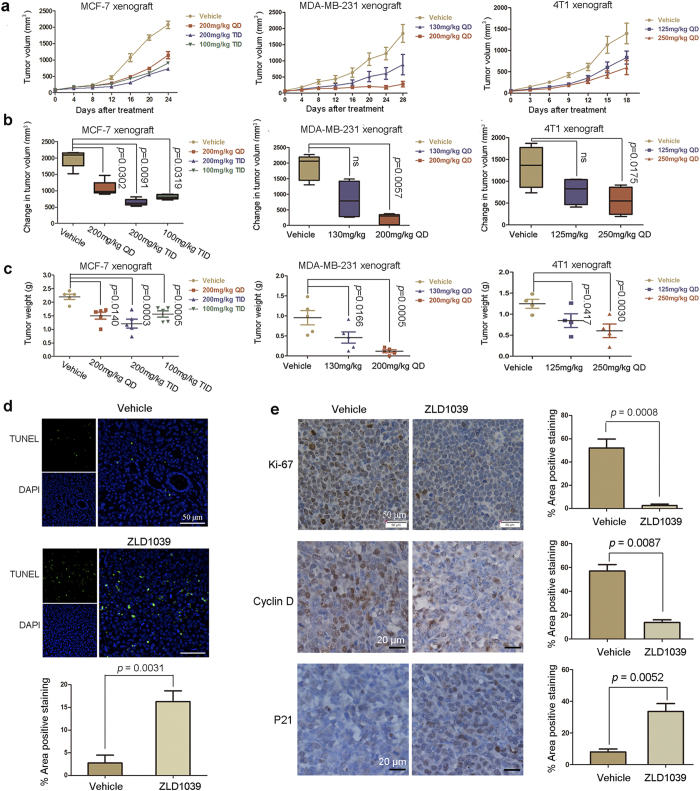
Figure 6. Antitumor efficacy of ZLD1039 in vivo.
(a) Tumor regression of MCF-7, MDA-MB-231, or 4T1 tumor xenografts in mice treated with different dosing schedules of ZLD1039 three times a day (TID) or once a day (QD). Data are tumor volume mean ± SEM (n = 5). (b) Quantitative analysis of tumor volume change on final study day. Boxes display lower (25th) and upper (75th) quartiles with a line at the median; whiskers extend from minimum to maximum observation. P-values were determined using two-tailed Student’s t-test; ns, not significant. (c) Represented weight of tumors from mice in different groups. Data are mean ± SEM. P-values comparing two groups were determined using two-tailed Student’s t-test. (d) After 14 days treatment, MCF-7 tumors treated with 200 mg/kg ZLD1039 or vehicle were examined using terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labelling (TUNEL) assay (n = 3). TUNEL-positive cells were counted in four high power fields/slide, and data were summarized as a percentage of positive cells. Data are mean ± SD. P-values were determined using two-tailed Student’s t-test. (e) Tumor tissues from MCF-7 xenografts treated with vehicle or ZLD1039 (200 mg/kg) for 14 days were immunohistochemically analysed with anti-Ki67, anti-cyclin D, and anti-P21 antibodies (n = 3). Representative images and quantitative analysis of percentage of positive staining are shown. P-values for comparing two groups were determined using two-tailed Student’s t-test.