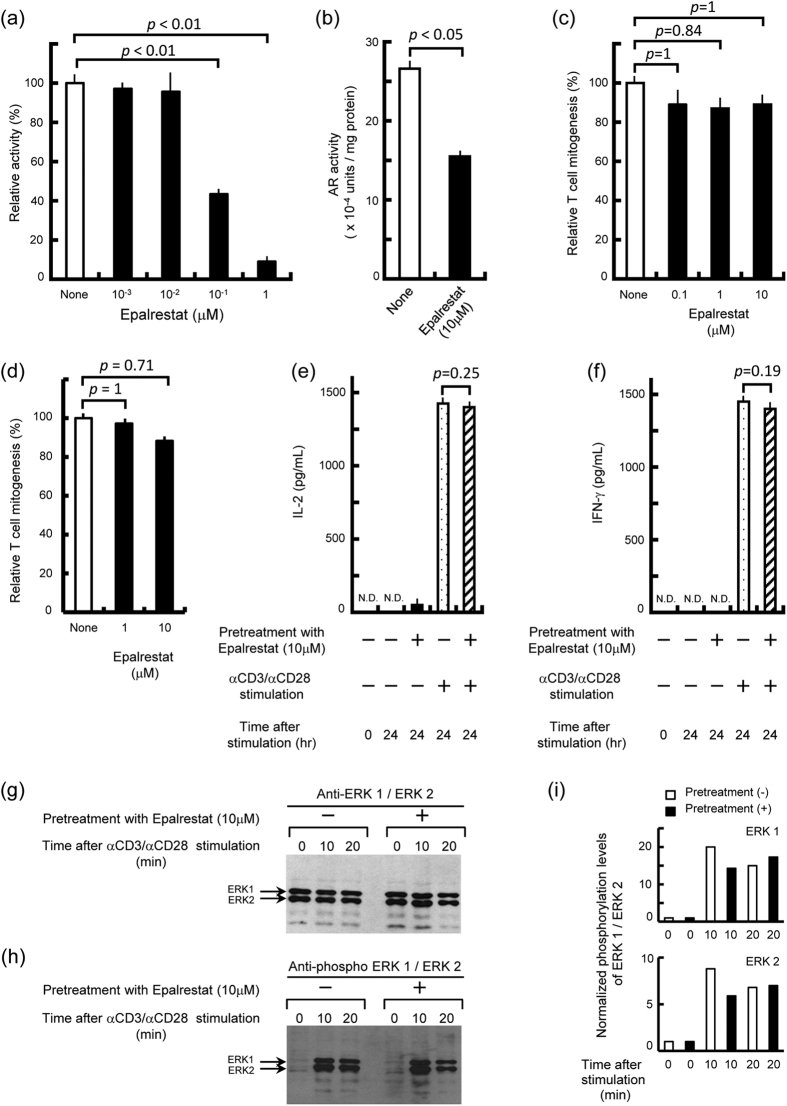
Figure 3. Evidence that enzymatic activity of AR is not essential for signaling pathways of T cells leading to the activation of T-cell functions.
(a) Inhibitory effects of epalrestat against enzymatic activity of recombinant human AR. (b) Effects of epalrestat on the cellular AR activity when D10.G4.1 mouse Th2 cells were treated with 10 μM epalrestat. (c–f) Effects of the AR inhibitor epalrestat on the proliferative response (c, d), IL-2 production (e), and IFN-γ production (f) of mouse splenic T cells induced by anti-CD3 and anti-CD28 Abs (c, e, f) or Con A (d). The inhibition of enzymatic activity of AR did not affect the T-cell proliferative response or IL-2 and IFN-γ expressions responding to TCR stimulation. (g, h) Effects of epalrestat on the cellular levels of ERK1/2 expression (g) and phosphorylation of ERK1/2 (h) in splenic T cells before and after stimulation with anti-CD3 and anti-CD28 Abs. The inhibition of AR activity had no significant influence on the expression and phosphorylation profiles of ERK1/2 in T cells undergoing TCR stimulation. (i) Normalized phosphorylation levels of ERK1/2 in T cells before and after TCR stimulation. There was no significant change due to inhibition of the enzymatic activity of AR in the levels of phosphorylation of ERK1/2 per molecule.