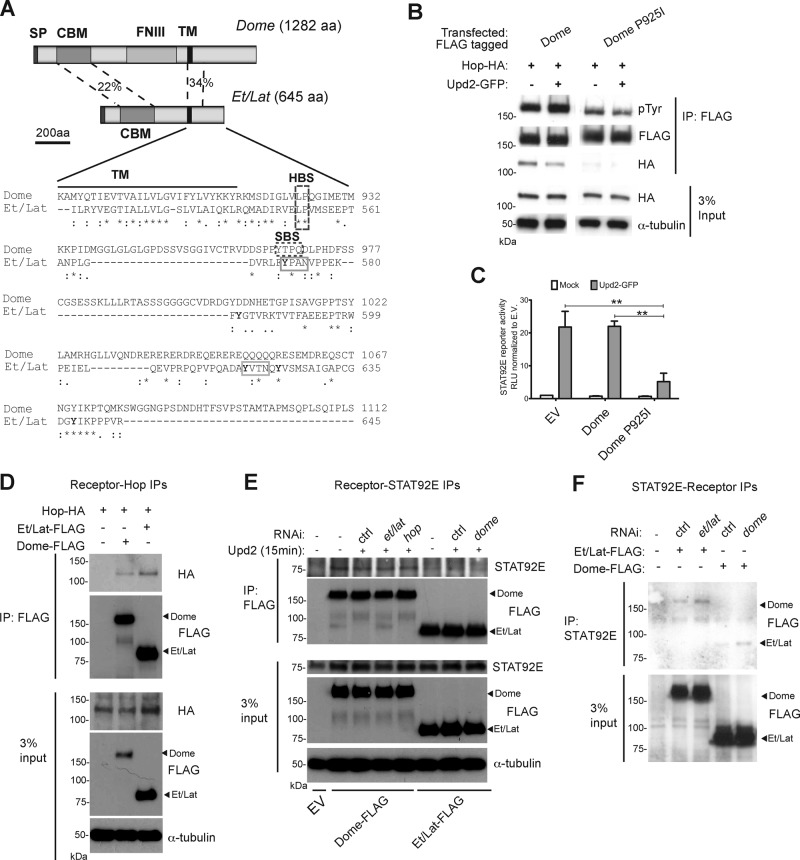
FIGURE 2:
Et/Lat can bind to intracellular JAK/STAT components. (A) Visualization of Dome and Et/Lat protein domain structure. Clustal sequence alignment for intracellular portion of Dome and Et/Lat. Transmembrane domain (TM; black bar), putative binding sites for Hop (HBS), and STAT92E (SBS) are highlighted. Tyrosine residues in Et/Lat are shown with bold letters. SP, signal peptide; CBM, cytokine-binding motif; FNIII, fibronectin 3 domains; TM, transmembrane domain; HBS, Hop-binding site; SBS, STAT binding site; aa, amino acids). Percentage identity between the indicated domains. (B) Dome-FLAG can coimmunoprecipitate Hop-HA, but this is greatly reduced when the putative Hop-binding site is mutated (Dome P925I). Tyrosine phosphorylation (pTyr) of Dome is also reduced in the Dome-P925I. (C) STAT92E transcriptional reporter activity assay. JAK/STAT pathway signaling is greatly reduced in cells expressing Dome P925I even in the presence of Upd2 ligand, **p < 0.01. (D) Immunoprecipitation assays show that both Dome-FLAG and Et/Lat-FLAG can coprecipitate Hop-HA. (E) Immunoprecipitation assays after transfection of the indicated Dome-FLAG and Et/Lat-FLAG proteins show that both proteins can coprecipitate endogenous STAT92E. dsRNA targeting the labeled mRNAs was added as indicated; ctrl, a nontargeting dsRNA. Knockdown of endogenous Dome (final lane) did not disrupt the ability of Et/Lat to bind to STAT92E. Upd2-conditioned medium was added for 15 min before protein harvesting. EV, empty vector. (F) Immunoprecipitation of endogenous STAT92E from cells expressing FLAG-tagged Dome or Et/Lat shows that both receptors are coprecipitated. Treatment with dsRNAs targeting the endogenous receptors suggests that interactions detected are independent of potential heterodimer formation.