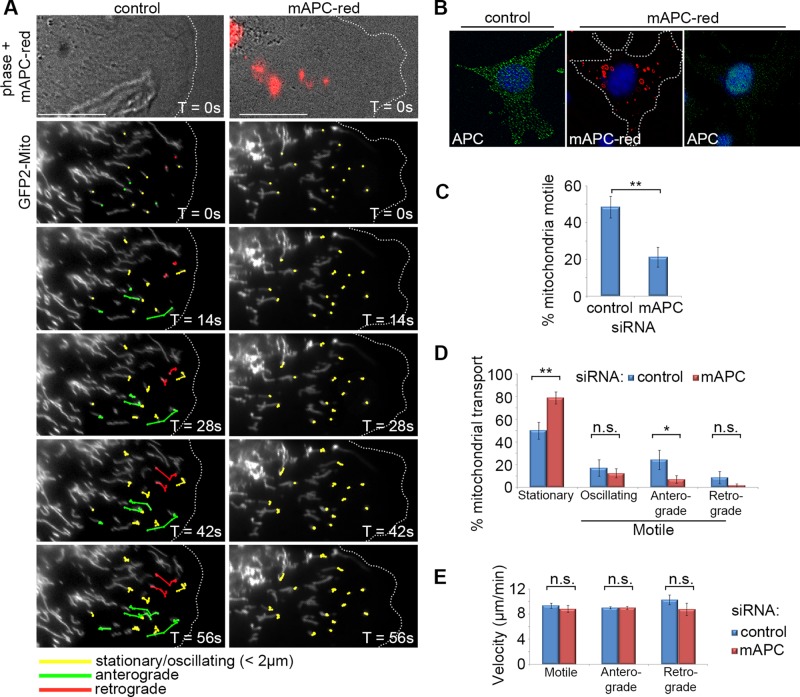
FIGURE 4:
Loss of APC reduces initiation of mitochondrial transport but not velocity in NIH 3T3 cells. Cells were treated with either control or red fluorescently tagged mouse APC siRNA (mAPC-red), transfected with a mitochondrial marker (pGFP2-Mito), and then grown to confluence. Cells were then wounded and were analyzed 5 h later by live-cell time lapse, with images captured every 7 s for a total of 5 min. Post image acquisition, mitochondria were selected at random for tracking manually using the ImageJ plug-in MTrack. (A) Representative cell images are shown from ∼1 min after commencement of tracking. Mitochondria are marked for different types of movement: stationary/oscillating (yellow), anterograde/membrane-directed (green), and retrograde/nucleus-directed (red). The majority of directed movement of mitochondria was blocked when APC was silenced (successful APC silencing indicated by red fluorescence). Scale bars: 10 μm. (B) Confirmation of APC knockdown in fixed cells treated with mAPC-red siRNA (C) Analysis of mitochondrial motility revealed that loss of APC resulted in decreased overall mitochondrial transport (**, p < 0.01). (D) This was particularly evident in the anterograde direction (*, p < 0.05; n.s., not significant). (E) Analysis of mitochondrial velocity (μm/min) revealed that loss of APC had no impact on the speed of movement in either direction once transport was initiated (n.s., not significant). n over three independent experiments: control cells, n = 18, and mitochondria, n = 202; mAPC-red cells, n = 18, and mitochondria, n = 189. Bar graph data presented as mean (±SD), statistical analysis by two-tailed t test (C–E).